Cambio Ion Exchange Agaroses
Ion exchange chromatography is a standard method to purify proteins according to their surface charge. Chromatography matrices that provide a positively or negatively charged surface are used for separation. This method is well established and does not require any affinity tags to be fused to the protein to be purified. Therefore it is suitable for the purification of both native and recombinant proteins.
Cambio Agaroses are based on BioWorks Workbeads, providing highest binding capacities and reproducible results, both in batch and FPLC chromatography applications.
Cambio Ion Exchange Agaroses provide:
- high protein yields
- reproducible protein purifications
- weak and strong anion and cation exchangers available
- Ideal in combination with Hydrophobic Agaroses
Anion and cation exchangers
Ion exchange chromatography media are classified by their surface properties into four categories: anion and cation exchangers, and weak and strong exchangers.
Anion exchangers are positively charged and serve to purify proteins with an overall negatively charged surface. Cation exchangers, on the other hand, are negatively charged and are used to purify proteins with an overall positive charge. See Fig. 1 for an overview.
|
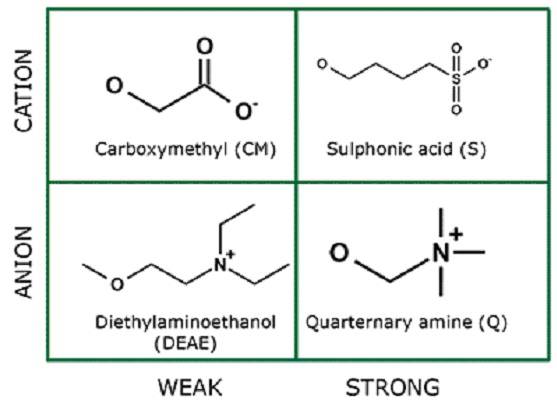
Fig. 1: Chemical structures of different functional groups used in ion exchange matrices.
What is the best matrix to purify my protein?
The overall charge of a protein depends on its isolelectric point (pI) which is defined as the pH at which the surface charge of the protein is zero. It can be theoretically determined by the charge sum of all amino acids in the protein. However, this theoretical value might differ considerably from the actual value because many amino acid residues will be folded inside the protein and not exposed to the surface. Experimental determination of pI is usually done using isoelectric focusing (IEF) gel electrophoresis. A given protein will be protonated (and hence positively charged) at pH values below its pI, and deprotonated (negatively charged) at pH values higher than its pI. The actual pH used for purification should be chosen with regard to highest protein stability and activity.
"Weak" and "strong" ion exchangers
The terms "weak" and "strong" do not refer to the yields or purity levels that can be achieved with a certain material, but rather on the behaviour of the matrices towards changes in pH. Weak ion exchangers (DEAE and CM) are more readily protonated by the surrounding buffer than strong ion exchangers, and therefore show a variation in surface properties depending on pH. Strong ion exchangers (Q and SP), on the other hand, are not influenced by pH and retain their full ionization over a broad pH range.
Strong ion exchangers (Q and SP) are typically used for initial development and optimization of purification protocols. Weak ion exchangers (DEAE and CM) are more flexible in terms of selectivity and a good option for proteins that are unstable and require mild elution conditions. In general, elution conditions depend on protein stability, but in many cases, simple molecules such as NaCl can be used for elution. To increase the purity of the resulting elution fraction, concentration gradients are used rather than stepwise elutions.
|
|
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200