Cambio Hydrophobic Interaction Agaroses
Hydrophobic interaction chromatography (HIC) is a well-established method to purify proteins according to their surface charge and hydrophobicity. Chromatography matrices that provide a hydrophobic surface are used for separation. This method does not require any affinity tags to be fused to the protein to be purified. Therefore it is suitable for the purification of both native and recombinant proteins.
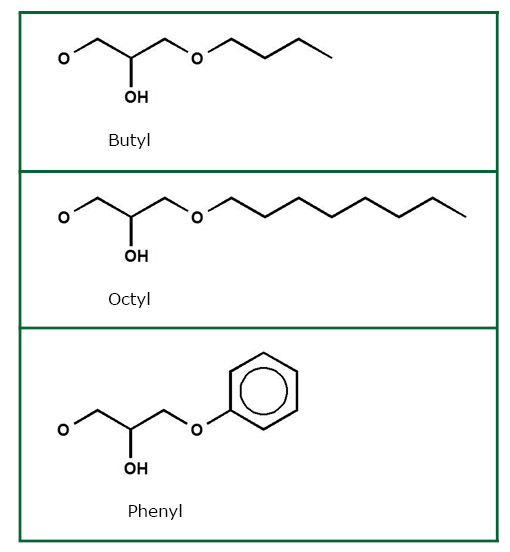
We offer hydrophobic interaction matrices, with phenyl, octyl, or butyl groups on the surface. For a novel protein, comparing different matrices is strongly recommended, and a HIC screening set with small quantities of each matrix is available.
Our Cambio HIC Agaroses are based on BioWorks Workbeads, providing highest binding capacities and reproducible results, especially in FPLC chromatography applications.
Cambio Hydrophobic Interaction Agaroses provide:
- Increased ligand density for high protein yields, especially for smaller proteins
- Choice of different matrices: Phenyl, Octyl, and Butyl Agarose
- Homogeneous base matrix made of 40 µm diameter, crosslinked agarose
- Ideal as second step after purification with Ion Exchange Agaroses
Hydrophobic interaction (HIC)
HIC separation is based on the interaction of hydrophobic regions of a protein with chromatography media that contain hydrophobic groups such as butyl, octyl, or phenyl on the surface. This interaction is enhanced by high ionic strength, making HIC an ideal purification step after ammonium sulfate precipitation or after elution from ion exchange media in high salt buffers.
Binding
Proteins might precipitate at very high salt concentrations, therefore it is recommended to test in small scale experiments if the protein of interest stays in solution at the salt concentration required for binding.
Elution
Bound proteins can be eluted with low-salt buffers. It is highly recommended to use a salt gradient (e.g. ammonium sulfate or sodium chloride) for elution to ensure that the protein is kept in the optimal salt concentration required for stability.
Which matrix for my protein?
In contrast to isoelectric point determination, it is difficult to predict a protein's overall hydrophobicity, as only isolated patches on the protein surface may be hydrophobic but sufficient for interaction with the HIC matrix. Therefore, it is recommended to test different resins for their suitability to bind the protein of interest at concentrations that keep the protein in solution, elute it at salt concentrations suitable for protein stability and activity, while separating it from contaminating proteins. We have developed the Cambio HIC Starter set for easy comparison of the three different matrices: butyl, ocytl and phenyl resins.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200