The Stealthy Invader - How to Detect a Mycoplasma Contamination?
Friday, 21 June 2024
Different Methods for Mycoplasma Detection
Mycoplasma contamination remains a persistent
challenge in cell culture laboratories and the biopharmaceutical
industry. Detection of mycoplasma contamination is crucial for
maintaining the reliability and reproducibility of cell-based
experiments and is essential to ensure the safety and quality of
biopharmaceutical products. Since mycoplasma are invisible to the naked
eye and do not cause turbidity in the growth medium it is difficult to
detect a contamination.
This article provides an overview of various
methods available for mycoplasma detection, highlighting their
principles, advantages, and limitations.
Culture-based Methods
Mycoplasma can be detected by a culture-based
method. This compendial method is the gold standard and is described in
the European, United States and Japanese Pharmacopeia. Two test methods
are used - the culture method and the indicator cell culture method.
Culture Method
The test sample is inoculated directly into a
broth medium. The liquid cultures are subcultured at multiple time
points onto agar plates to visualize mycoplasma growth. At the end of
the incubation period, the presence of mycoplasma colonies is examined
by microscope. The total test takes at least 28 days to produce a
negative test result.
Indicator Cell Culture Method
Not all mycoplasma strains can be successfully
cultured by the conventional culture method because they do not grow in
the standard media. The indicator cell culture test is performed by
transferring the sample into a Vero cell culture. After 3-5 days
incubation the indicator cells are stained with Hoechst DNA stain and
examined under the microscope for surface fluorescence. This method is
faster than the culture-based method but is less sensitive and still
takes several days to complete. [1], [2]
DNA Staining
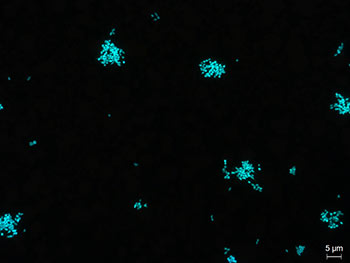 Stained mycoplasma cells
Stained mycoplasma cells
A simple way to test for the presence of
Mycoplasma in your cell culture is DNA staining e.g. with DAPI
(4',6-diamidino-2-phenylindole) or Hoechst. DAPI intercalates strongly
into the minor groove of DNA and shows a blue staining that can be
visualized by UV excitation (460 nm) using a fluorescence microscope. In
mycoplasma-free cell cultures, only cell nuclei are labelled. If
mycoplasma cells are present their DNA will be labelled too.
Although DNA staining is an easy and rapid test,
sometimes interpreting the results can be difficult and some experience
is definitely necessary. Due to their small size, single mycoplasma
cells cannot be detected under fluorescence microscope. This means that
only a high degree of contamination leads to a visible obscure veil
formation, which may not be clearly identifiable. Furthermore, if the
condition of cell culture is not good, the DNA staining results can be
misinterpreted. [1]
Polymerase Chain Reaction
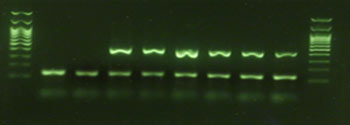 Exemplary agarose gel image
Exemplary agarose gel image
In some cases, a
faster method is required, especially if the product to be released has a
short shelf life (e.g. ATMPs). The majority of rapid mycoplasma
detection methods are PCR-based methods, which are accepted by
regulatory authorities as an alternative to conventional culture-based
methods.
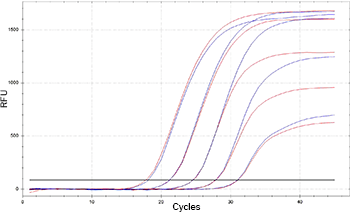 Exemplary qPCR amplification plot
Exemplary qPCR amplification plot
Mycoplasma are specifically detected by
amplifying a highly conserved 16S rRNA coding region in the mycoplasma
genome. PCR-based methods are highly sensitive compared to culture-based
or staining methods. It is possible to detect mycoplasmas in cell
cultures in a very early state of contamination and low concentrations.
Compared to the culture or staining method, PCR analysis delivers
accurate and highly sensitive results in a few hours. Mycoplasma can be
detected via conventional PCR and subsequent gel electrophoresis, via
real-time PCR or digital PCR. These easy, rapid, efficient and
cost-effective methods deliver highly sensitive, specific and reliable
results.
 Minerva Biolabs launched the world’s first mycoplasma PCR assay validated according to Ph. Eur. 2.6.7 in 2006.
Minerva Biolabs launched the world’s first mycoplasma PCR assay validated according to Ph. Eur. 2.6.7 in 2006.
Other methods
Other technologies
have also been used to rapidly detect mycoplasma contaminations, such
as ELISA, fluorescence in situ hybridization (FISH) or bioluminescence
assays. These methods have largely been superseded by PCR-based methods.
Therefore, we have only compared the culture method, DNA staining and
PCR method below:
Different Mycoplasma Testing Methods
Gold standardSpecific
RapidCost-effective
Interpretation of results can be difficult, especially if cell culture isin poor conditionExperience is necessary
No discrimination between live and dead cellsNo living mycoplasmas required for positive control & validation
Rapid (few hours)Broad detection rangeLittle hands-on timeHighly sensitive and specific
Takes 28 daysLabour-intensiveRequires living mycoplasma cellsfor positive control & validation
Culture method
DNA staining
PCR | qPCR | dPCR
Advantages
Disadvantages
Discover our PCR-based detection kits as an
alternative to costly, time-consuming culture-based methods, delivering
accurate and highly sensitive results in about 3 hours.
Venor® GeM Mycoplasma Detection kits
• Easy-to-use
• Rapid turnaround time
• Detect >100 relevant mycoplasma species
• Highly sensitive
• Ready-to-use lyophilized kit components simplify logistics and storage
• TaqMan® probes ensure the highest level of PCR specificity
• Non-infectious mycoplasma standards (10 or 100 CFU) available
Select between
• Conventional, real-time or digital PCR
• Screening in R&D or release testing of biopharmaceuticals (acc. to EP 2.6.7, USP<63>, JP G3)
Would you like to try first? We offer samples for many of our mycoplasma PCR kits free of charge.
Please find sample request information on the appropriate product page.
[1] Nikfarjam L,
Farzaneh P. Prevention and detection of Mycoplasma contamination in cell
culture. Cell J. 2012 Winter;13(4):203-12. Epub 2011 Dec 22. PMID:
23508237; PMCID: PMC3584481.
[2] Drexler HG, Uphoff CC.
Mycoplasma contamination of cell cultures: Incidence, sources, effects,
detection, elimination, prevention. Cytotechnology. 2002
Jul;39(2):75-90. doi: 10.1023/A:1022913015916. PMID: 19003295; PMCID:
PMC3463982.
Article Source:
Minerva Biolabs GmbH