Latest Changes in Pyrogen and Endotoxin Testing
Wednesday, 25 September 2024
Why is Pyrogen Testing Necessary?
In the pharmaceutical industry, the safety of
products is paramount, particularly when it comes to preventing adverse
reactions in patients. One critical aspect of safety testing is pyrogen
detection. Pyrogens are substances that can induce fever when introduced
into the body. They can be either microbial, such as endotoxins from
bacteria, or non-microbial, originating from various sources like
chemicals or contaminants. Complete pyrogen testing is essential for
ensuring that pharmaceutical products, especially those administered
intravenously, are free from these harmful substances.
What are Pyrogens and How are they Classified?
Pyrogens are fever-inducing substances primarily
derived from microorganisms such as bacteria, viruses, yeasts, molds, or
chemical substances. They can cause severe reactions, including fever,
shock, and even death if not properly controlled. Pyrogens are generally
classified into two main types:
- Bacterial endotoxins are
the most potent pyrogenic contaminants and are ubiquitous. They derive
from the cell wall of Gram-negative bacteria and include
membrane-derived compounds such as lipid polysaccharide (LPS),
flagellin, peptidoglycans, lipids, proteins, etc. It´s difficult to
remove them during manufacturing since they often are heat stable.
- Non-Endotoxin Pyrogens (NEPs)
include substances from Gram-positive bacteria (e.g. peptidoglycans and
lipoteichoic acids), viruses, yeasts and molds as well as certain
chemical substances (e.g. primary packaging material, rubber, plastic or
metal abrasion).
How to Detect Endotoxins and Pyrogens?
There are a variety of methods that can be used
to detect pyrogens. They can be categorised in two groups: Complete
pyrogen tests and bacterial endotoxin tests. The following overview
shows main features and differences between these tests.
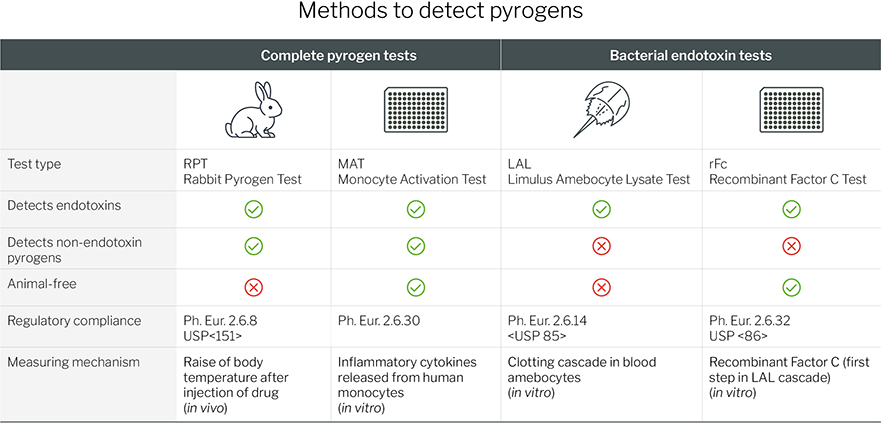
Bacterial Endotoxin Testing
Bacterial endotoxin tests (BET) only detect bacterial endotoxins and no NEPs. Traditionally, the
Limulus Amebocyte Lysate (LAL) test is used for endotoxin testing. This test relies on blue blood cells from the Atlantic horseshoe crab, Limulus olyphemus,
which clots in presence of endotoxins. In Asia the test is called TAL
test, because the blood cells of another horseshoe crab species, Tachypleus spp., are used. During blood collection many animals die.
What has been permitted in the European pharmacopeia since 2018 is now also permitted in the USA – The
recombinant Factor C (rFC) test has finally been accepted by the US pharmacopeia and will become official in May 2025 [1].
This test copies the LAL test and uses a synthetically produced,
recombinant version of the first enzyme in the LAL clotting cascade.
Since this test does not rely on the blood of horseshoe crabs, no
animals have to suffer and die for it.
Complete Pyrogen Testing
When it comes to complete pyrogen testing, traditionally, the Rabbit Pyrogen Test (RPT)
has been the standard method for detecting pyrogens. In this test, the
product is injected into rabbits, and their body temperature is
monitored for any rise indicative of a pyrogenic response.
With increasing awareness of ethical concerns and
growing demand for animal-free alternatives, the Monocyte Activation
Test (MAT) was introduced into the European Pharmacopeia (Ph. Eur.) in
2010 – providing a human in vitro system to detect and quantify pyrogenic substances.
In June 2024 the Ph. Eur. Commission has decided to end the RPT era
by deleting the RPT from the Ph. Eur.. The revised texts omitting the
RPT and the new general chapter on
Pyrogenicity (5.1.13) will be implemented in Supplement 11.8 of the Ph. Eur. on 1 July 2025 [2]. This represents a significant milestone for animal welfare and the progress of modern in vitro
methods for pyrogen testing. Consequently, the RPT will no longer be
mandated in any chapter of the Ph. Eur.. Pharmaceutical manufacturers
are now responsible to select and establish a suitable in vitro test method (e.g. MAT) to monitor the pyrogenicity of their products.
The MAT is based on the
activation of monocytes by pyrogenic substances present in the sample.
Upon exposure to potential pyrogens, monocytes undergo a signalling
pathway resulting in secretion of pro-inflammatory cytokines, such as
IL-1β, TNF-α and IL-6. The release of these cytokines is measured in the
MAT.
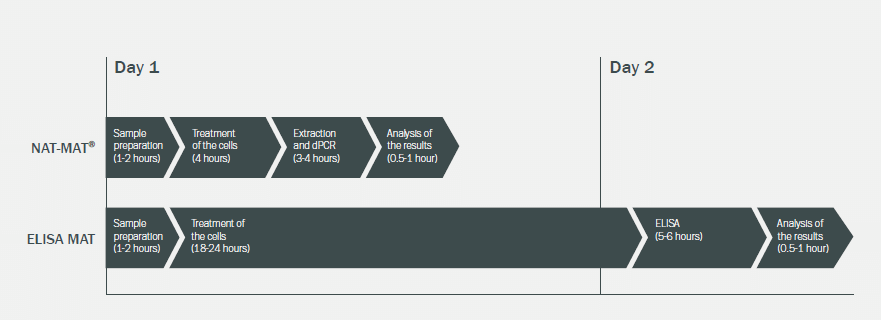
Discover Next-Generation MAT Testing
Minerva Biolabs has developed a next generation
MAT system that measures the gene expression of IL-1β and TNF-α using
digital PCR –
the NAT-MAT®. The protocol is
optimized for fast and reliable pyrogen detection and offers robust and
highly sensitive results. The NAT-MAT® enables complete pyrogen testing
and detects endotoxin as well as non-endotoxin pyrogens. The test can be
conducted as in-process control and as final QC release testing of
medicinal products according to Ph. Eur. 2.6.30. Due to the parallel
measurement of two cytokines and one housekeeping gene more accurate
results can be generated. The housekeeping gene also works as quality
control of extraction and cell number, thus the assay functions within a
range of cell densities. Analysis of the results can be done
automatically by Minerva Biolabs' NAT-MAT® software according to the
requirements of Ph. Eur. 2.6.30.
In contrast to ELISA-based MATs, our NAT-MAT® can be performed within 1 day.
[1] https://www.usp.org/news/expert-committee-approves-endotoxin-testing-using-non-animal-derived-reagents[2] https://www.edqm.eu/en/-/ph.-eur-bids-adieu-to-rabbit-pyrogen-test-in-its-monographs
Article Source:
Minerva Biolabs GmbH