TransforMax™ EPI300™ E. coli cells lack the tonA gene and are engineered for use with EPICENTRE's CopyControl™ cDNA, Gene, and PCR Cloning Kit and other CopyControl Cloning Systems* that do not require phage T1-resistant cells. The cells contain an inducible mutant trfA gene whose gene product is required for initiation of replication from the oriV origin of replication, such as that in CopyControl pCC1™ vectors or in clones that are retrofitted with CopyControl capability using the EZ-Tn5™<oriV/KAN-2> Insertion Kit. On LB chloramphenicol plates or in LB or SOC media supplemented with chloramphenicol, CopyControl clones grown in TransforMax EPI300 E. coli replicate at single-copy number from the F-factor replicon because expression of the trfA gene is repressed. Addition of CopyControl Induction Solution induces the cells to express the trfA gene product and induces their replication at high-copy number from oriV (Fig. 1). Replication from oriV results in higher yields and higher purity of cloned DNA.
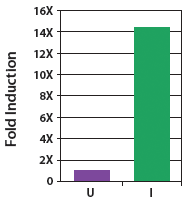 |
| Figure 1. Copy number of CopyControl™ BAC clones can be induced 10- to 20-fold in TransforMax™ EPI300™ E. coli. The yield of BAC DNA from CopyControl BAC clones of 110-145 kb increased >14-fold following addition of CopyControl Induction Solution. U=uninduced cells; I=induced cells. |
Genotype
F- mcrA Δ(mrr-hsdRMS-mcrBC) Φ80dlacZΔM15 ΔlacX74 recA1 endA1 araD139 Δ(ara, leu)7697 galU galK λ- rpsL (StrR) nupG trfA dhfr
TransforMax EPI300 Electrocompetent E. coli
- Transformation efficiency of >1 x 1010 cfu/µg of pUC19.
TransforMax EPI300 Chemically Competent E. coli
- Transformation efficiency of >5 x 108 cfu/µg of pUC19.
For use with vectors or clones having an inducible oriV for CopyControl™ capability.
*Covered by issued and/or pending patents.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200