2'-F-I-CE Phosphoramidite

5'-Dimethoxytrityl-deoxyInosine, 2'-fluoro-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
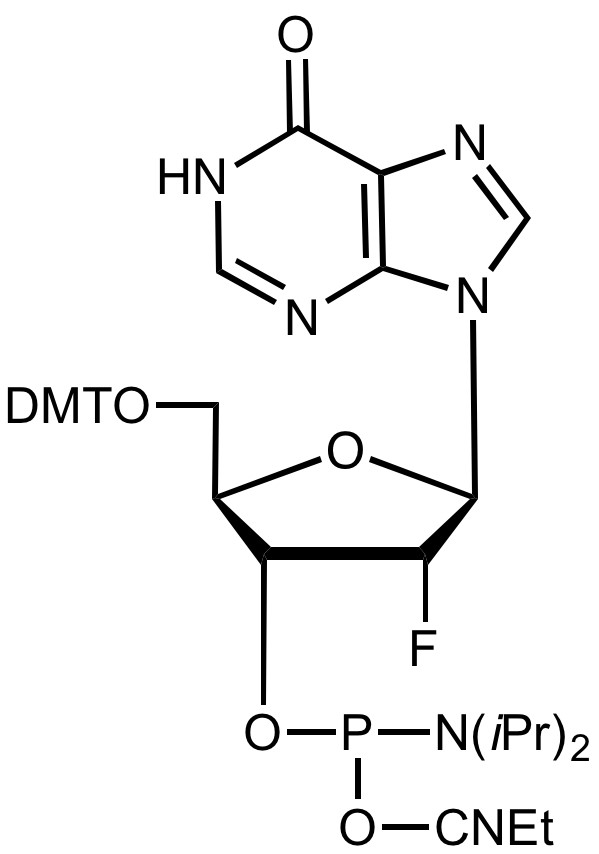
Product Specifications
F.W.:
332.18
Description
The significance of inosine
in oligonucleotide synthesis comes from its ability to base pair with
each of the standard bases (A, C, G, and T/U). Due to these unique
pairing properties, inosine is considered as a “Universal Base” and has
been an attractive candidate for many
applications involving either synthetic primers or probes. This 2’-F
version of inosine can be used in the same way as standard 2’-F
phosphoramidites.
Details
Usage
- Coupling: 3 minutes coupling time recommended.
- Deprotection:
Ammonium Hydroxide for 17 hours at 55°C or 30% Ammonia Hydroxide/40%
Methylamine 1:1 (AMA) for 2 hours at Room Temperature. (Note - heating
in AMA will lead to some degradation of the 2'-Fluoro nucleotides.)
Safety Data Sheet
Glen Report 32.13: New Product — 2’-Fluoro-Inosine-CE Phosphoramiditer
| Specifications |
|---|
| Diluent |
Anhydrous Acetonitrile |
| Storage |
Freezer storage, -10 to -30°C, dry |
| Stability | 1-2 Days |
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200