| 5-Me-dC Brancher Phosphoramidite |
Catalog Number: 10-1018-xx
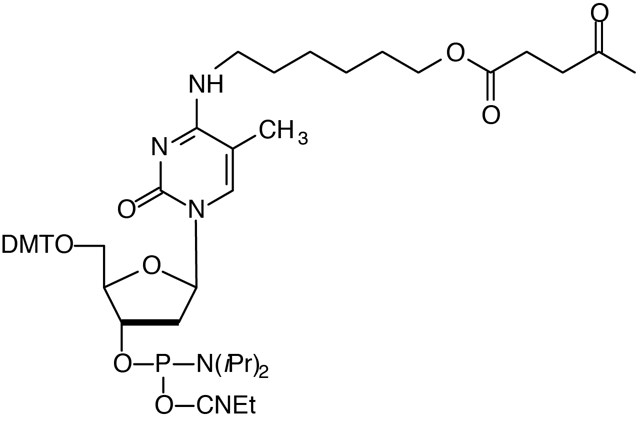
Description: 5-Me-dC Brancher Phosphoramidite
5'-Dimethoxytrityl-N4-(O-levulinyl-6-oxyhexyl)-5-Methyl-2'-deoxyCytidine,
3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C51H68N5O10P |
M.W.: 942.10 |
F.W.: 402.36 |
Diluent: Anhydrous Acetonitrile |
| Coupling: dC Brancher reacts in a manner identical to normal phosphoramidites. |
| Levulinyl Deprotection: The levulinyl protecting group can be selectively removed without cleavage of the oligonucleotide from the CPG by treatment with 0.5 M Hydrazine hydrate in 1:1 pyridine/acetic acid. [Note hydrazine hydrate is a violent poison that is both volatile and readily absorbed through skin] Fit the column with syringes and push the solution back and forth across the column. Let sit for 15 minutes at room temperature. Rinse the column with 1.5 mL of 1:1 pyridine/acetic acid (3x) and then 1.5 mL of ACN (3x). Dry CPG under a stream of argon and proceed with the synthesis of the branching sequence. If a non-branching control is desired, simply deprotect in ammonium hydroxide as required by the nucleobases. Technical Bulletin |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 24 hours |
BRANCHING PHOSPHORAMIDITE
A branching monomer is required to construct comb-like oligonucleotide probes. The developers of the comb system from Chiron Corporation evaluated3 several protecting groups for the branch point and chose levulinyl (LEV), which is specifically removed using a reagent containing hydrazine hydrate, acetic acid and pyridine.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200