| ATG Trimer Phosphoramidite |
Catalog Number: 10-1032-xx
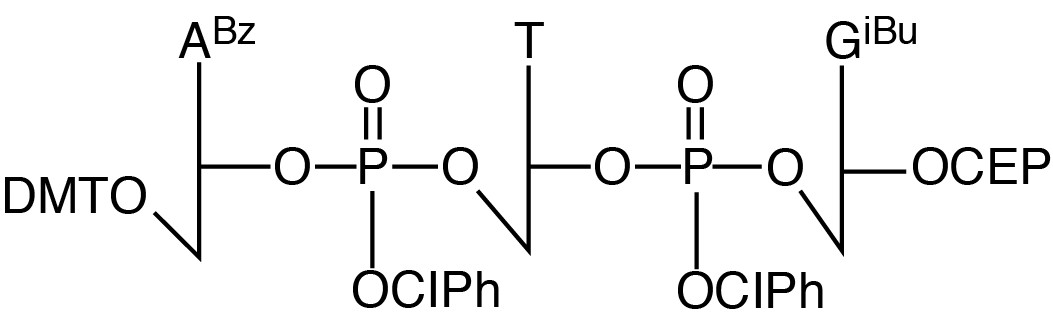
Description: ATG Trimer Phosphoramidite
| 5'-Dimethoxytrityl-N6-benzoyl-2'-deoxyAdenosine-3'->5'-o-chlorophenyl-phosphoryl-2'-deoxyThymidine-3'->5'-o-chlorophenyl-phosphoryl-N2-isobutyryl-2'-deoxyGuanosine-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C83H89Cl2N14O21P3 |
M.W.: 1780.5 |
CAS number 1446702-57-3 |
All of the trimers are available individually so the researchers can
prepare custom trimer mixes. Two pre-made catalog trimer mixes are
available: 13-1991-xx, for incorporating all 20 amino acid codons
equally into a sequence and 13-1992-xx, for incorporating 19 amino acid
codons (-Cys). For a custom trimer mix of a particular subset of codons
or a trimer mix that represents a set of trimers that is biased toward a
particular codon or codons, please contact us for
a quotation and projected delivery date.
Usage
- Coupling: 15 minute coupling time recommended.
- Deprotection: 30% NH4OH for 17 hours at room temperature followed by an additional 4 hours at 55°C.
| Specifications |
|---|
| Diluent | Anhydrous Acetonitrile/Dichloromethane 1:3 (v/v) |
| Recommended Storage | Freezer storage, -10 to -30°C, dry |
| Stability In Solution | 2-3 days |
ABI 392/394
| Catalog # | Pack Size | Grams/Pack | 0.1M Dil. (mL) | Approximate Number of Additions |
|---|
| LV40 | LV200 | 40nm | 0.2μm | 1μm | 10μm |
|---|
| 13-1032-90 | 100 µmol | .178grams | 1 | 20 | 12 | 7.5 | 5.45 | 4 | 1 |
| 13-1032-95 | 50 µmol | .089grams | 0.5 | 3.33 | 2 | 1.25 | 0.91 | 0.67 | 0.17 |
Expedite
| Catalog # | Pack Size | Grams/Pack | Dilution (mL) | Approximate Number of Additions |
|---|
| Molarity | 50nm | 0.2μm | 1μm | 15μm |
|---|
| 13-1032-90 | 100 µmol | .178grams | 1.5 | 0.07 | 23.6 | 14.75 | 10.73 | 1.48 |
| 13-1032-95 | 50 µmol | .089grams | 0.75 | 0.07 | 8.6 | 5.38 | 3.91 | 0.54 |
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200