Miniaturized Ames Test
Miniaturized Ames Test – Principle
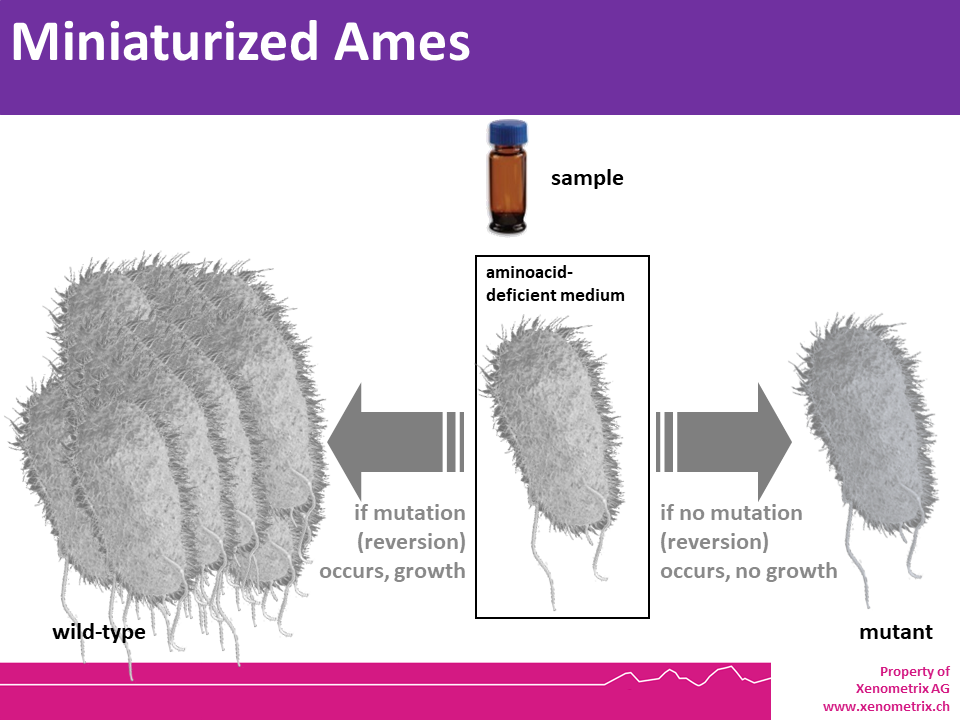
The principle of the miniaturized Ames
assays is identical to the agar based Ames Test OECD 471. Point
mutations were made in the histidine (Salmonella typhimurium) or the tryptophan (Escherichia coli) operon, rendering the bacteria incapable of producing the corresponding amino acid. These mutations result in his- or trp- organisms that cannot grow unless histidine or tryptophan is supplied.
A test sample’s mutagenic potential is
assessed by exposing these amino acid-requiring organisms to varying
concentrations of sample and selecting for the reversion event. Media
lacking histidine or tryptophan are used for this selection which allow
only those cells that have undergone the reversion to histidine /
tryptophan prototrophy to survive and grow. A mutagenic event (causing
either base substitutions or frameshift mutations) within the gene may
cause a reversion to amino acid prototrophy. These reverted bacteria
will then grow in histidine- or tryptophan-deficient media,
respectively, whereas non reverted bacteria will not be able to grow.
The media during the growth phase can be liquid or agar based.
Different Forms of miniaturized Ames test systems
- Miniaturized Ames Test “Ames MPF™ “ based on liquid microplate technology – 55 mg of test compound per 5 strains, /- S9
- Miniaturized Ames Test “MicroAmes24” based on 24 well agar plate – 30 mg of test compound per 5 strains, /- S9
- Ultra-miniaturized Ames Test “NanoAmes™” based on 25 square well agar plates – 35 ug of test compound per 5 strains, /- S9
- Ultra-miniaturized Ames Test “NanoAmes™ MPF” based on liquid microplate technology in development.
-
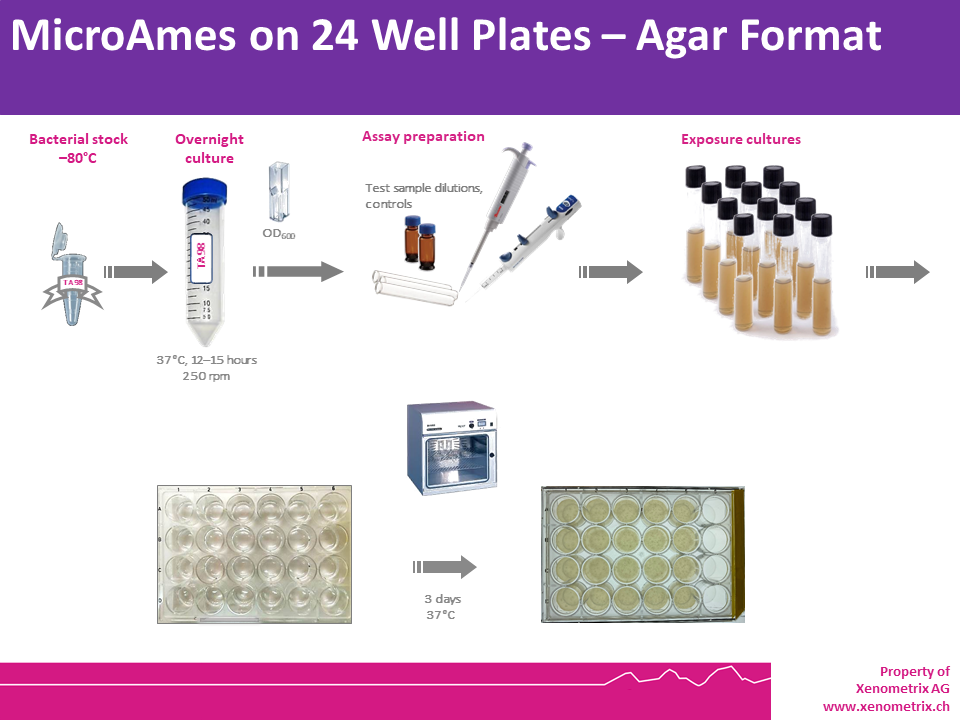
Miniaturized Ames Test in Agar Plates – Assay Description
Bacteria are exposed to different and
low concentrations of a test sample, as well as a positive and a
negative control in a medium containing limited quantities of histidine (S. typhimurium) or tryptophan (E. coli)
to support approximately two cell divisions. After exposure
(pre-incubation test) or without exposure (plate incorporation test),
The cultures in each condition (negative control, test samples and
positive controls) are poured on an agar support in multiwell plates (24
well plates) and are incubated. Reducing the surface area
(comparatively to a Petri dish), the amount of test sample needed to run
the test is dramatically reduced, while keeping similar limits of
detection.
Within three days, cells that have
undergone reversion to amino acid prototrophy will grow and form
colonies, whose counts will be compared to those grown in the solvent
(negative) control wells. Each dose is tested in triplicate to allow for
statistical analysis of the data.
A dose-dependent and significant
increase in the number of revertant colonies upon exposure to test
sample relative to the solvent controls indicates that the sample is
mutagenic.
The mutagenic potential of samples is
assessed directly, the compounds are tested in the absence and presence
of metabolic activation, provided by a liver homogenate, a
post-mitochondrial supernatant fraction S9, respectively.
*strongly recommended over Aroclor induced S9, Aroclor being a strong
environmental pollutant and on the EC list of banned chemicals.
More information on MicroAmes24 - Miniaturized 24-well agar plate Ames Test
More information on NanoAmes™ - Ultra-Miniaturized 25-well agar plate Ames Test
More information on S9
Miniaturized Ames Test in liquid 384 Well Microplate Format – Assay Description
Bacteria are exposed to 6 concentrations
of a test sample, as well as a positive and a negative control, for 90
minutes in medium containing enough histidine (S. typhimurium) or
tryptophan (E. coli) to support approximately two cell divisions. After
exposure, the cultures in each condition (negative control, test samples
and positive controls) are diluted in pH indicator medium lacking
histidine or tryptophan and aliquoted into 48 wells of a 384-well plate.
Within
two days, cells that have undergone reversion to amino acid prototrophy
will grow. In the liquid Ames II/Ames MPF system, bacterial metabolism
reduces the pH of the medium, changing the colour of the well the
bacteria are in. The number of wells containing revertant colonies are
counted for each dose and compared to a solvent (negative) control. Each
dose is tested in triplicate to allow for statistical analysis of the
data.
A dose-dependent and significant increase in the number of
revertant colonies upon exposure to test sample relative to the solvent
controls indicates that the sample is mutagenic.
The mutagenic
potential of samples is assessed directly, the compounds are tested in
the presence of metabolic activation, provided by a liver homogenate,
S9.
More information on Ames MPF™ Penta 2 – Ames Test in 384 well plates, liquid format
More information on Ames MPF™ 98/100 –2-strain Ames Test in 384 well plates, liquid format
Miniaturized Ames Tester Strains – Genotypes
E. coli Ames tester strains as well as S. typhimurium
Ames tester strains have been used for more than 40 years to detect
mutagenic compounds in chemicals, pharmaceuticals, copsmetics, biocides,
water and other environmental samples. They are all listed in the
guideline OECD 471: Bacterial Reverse Mutation Test and in guideline ICH
M7 for genotoxic impurities. Point mutations were made in the histidine
(Salmonella typhimurium) or the tryptophan (Escherichia coli)
operon, rendering the bacteria incapable of producing the corresponding
amino acid. These mutations result in his- or trp- organisms that
cannot grow unless histidine or tryptophan is supplied.
A test sample's mutagenic potential is
assessed by exposing these amino acid-requiring organisms to varying
concentrations of sample and selecting for the reversion event. Media
lacking histidine or tryptophan are used for this selection which allow
only those cells that have undergone the reversion to histidine /
tryptophan prototrophy to survive and grow. A mutagenic event causing
base substitutions or frameshift mutations within the gene may cause a
reversion to amino acid prototrophy. These reverted bacteria will then
grow in histidine- or tryptophan-deficient media, respectively, whereas
non reverted bacteria will not be able to grow. The media during the
growth phase can be liquid or agar based
E.coli WP2 pKM[101] strains are used in
agar based Ames Test, in miniaturized agar based (MicroAmes24) and
liquid format Ames Test (Ames MPF). The strains are used in accordance
with Guidelines OECD471, ICH M7.

More information on Ames Tester Strains.
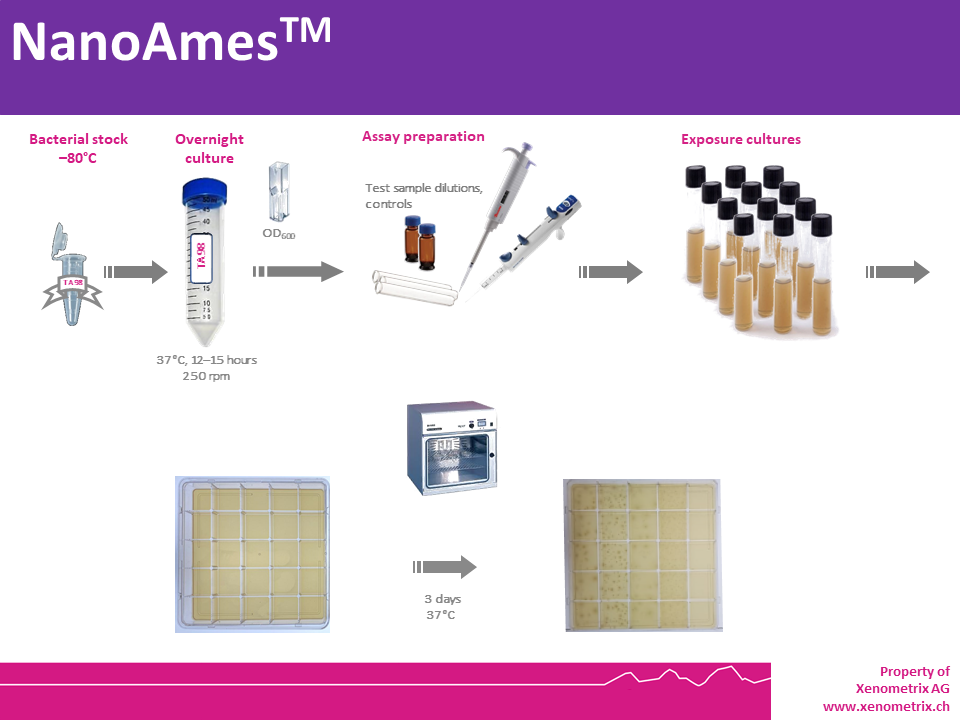
Ultraminiaturized NanoAmes™ – Assay Description
Bacteria are exposed to different and
ultralow concentrations (< 45 µg) of a test sample or a mixture, as
well as a positive and a negative control in a medium containing limited
quantities of histidine (S. typhimurium) or tryptophan (E. coli)
to support approximately two cell divisions. After exposure, the
cultures in each condition (negative control, test samples and positive
controls) are poured on an agar support in multiwell plates (25 -well
plates). Reducing the surface area (comparatively to a regular 12 cm
Petri dish) and adjusting the protocol, the amount of test sample needed
to run the test is dramatically reduced, while keeping similar limits
of detection.

Within three days, cells that have
undergone reversion to amino acid prototrophy will grow and form
colonies. In the ultraminiaturized Ames format NanoAmes (service
analytics available, kit in development) and NanoAmes MPF (kit in
development), revertant bacteria will form colonies, whose counts will
be compared to those grown in the solvent (negative) control wells. Each
dose is tested in quintuplicates to allow for statistical analysis of
the data.
A dose-dependent and significant
increase in the number of revertant colonies upon exposure to test
sample relative to the solvent controls indicates that the sample is
mutagenic.
The mutagenic potential of samples is
assessed directly and in the presence of metabolic activation, provided
by a liver homogenate, S9.
More information on NanoAmes™ - Ultra-Miniaturized 25-well agar plate Ames Test – in development.
Miniaturized Ames Test – References
- Brooks TM. 1995. The use of a streamlined bacterial mutagenicity assay, the MINISCREEN. Mutagenesis. 10(5):447–8.
- Burke DA, Wedd DJ, Burlinson B. 1996. Use of the Miniscreen assay to
screen novel compounds for bacterial mutagenicity in the pharmaceutical
industry. Mutagenesis. 11(2):201–5.
- Flamand N, Meunier J, Meunier P, Agapakis-Caussé C. 2001. Mini
mutagenicity test: a miniaturized version of the Ames test used in a
prescreening assay for point mutagenesis assessment. Toxicol In Vitro. 15(2):105–14.
- Cariou O, et al. in preparation.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200