| 2'-OMe-iPr-Pac-G-CE Phosphoramidite |
Catalog Number: 10-3621-xx
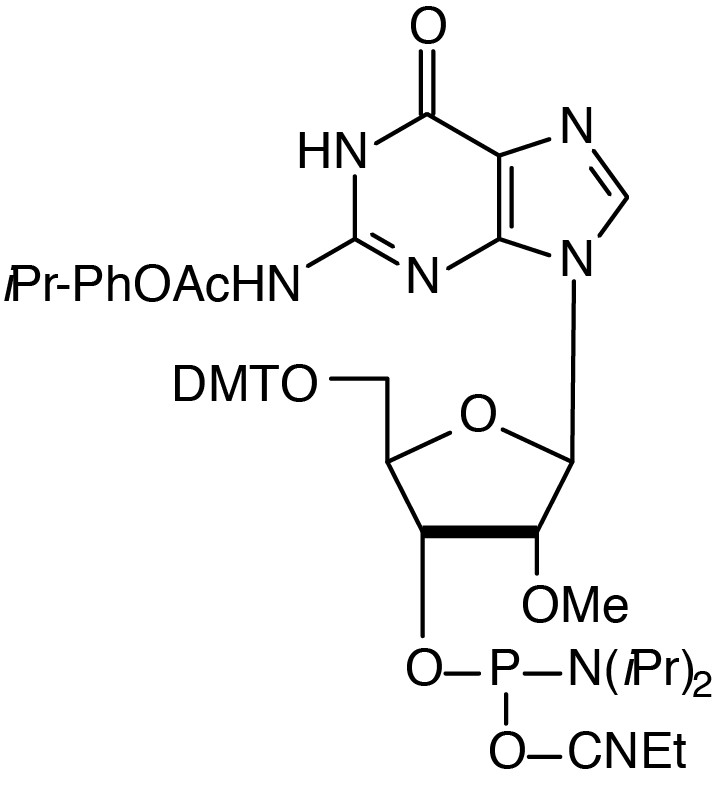
Description: 2'-OMe-iPr-Pac-G-CE Phosphoramidite
5'-Dimethoxytrityl-N2-isopropylphenoxyacetyl-Guanosine,2'-O-methyl,
3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C52H62N7O10P |
M.W.: 976.07 |
F.W.: 359.24 |
Diluent: Anhydrous Acetonitrile |
| Coupling: 6 minute coupling time. To avoid any exchange of the iPr-Pac group on the dG with acetyl, use the UltraMild Cap Mix A (40-4210-xx/ 40-4212-xx). |
| Deprotection: UltraMILD deprotection: 0.05M Potassium Carbonate in Methanol, 4 hours at Room Temperature OR 2 hours at Room Temperature in 30% Ammonium Hydroxide. Technical Bulletin |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: 2-3 days |
UltraMild 2’-OMe-RNA
The use of UltraMild monomers in oligonucleotide synthesis has allowed very sensitive dyes like TAMRA, HEX and Cy5 to be used virtually routinely. The DNA and RNA monomers are currently available and we also provide this set of 2’-OMe-RNA monomers. In our version of this chemistry, we use as protecting groups phenoxyacetyl (Pac) for A, acetyl (Ac) for C, and isopropyl-phenoxyacetyl (iPr-Pac) for G.
It has become clear that acetic anhydride in the conventional capping mix can cause transamidation in situations where an amine protecting group is quite labile. This leads to acetyl protection on the amino group that may be slow to be removed. Consequently, if many dG residues are included in the oligonucleotide, we recommend the use of phenoxyacetic anhydride (Pac2O) in Cap A. This modification removes the possibility of exchange of the iPr-Pac protecting group on the dG with acetate from the acetic anhydride capping mix
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200