Catalog Number: 10-1730-xx
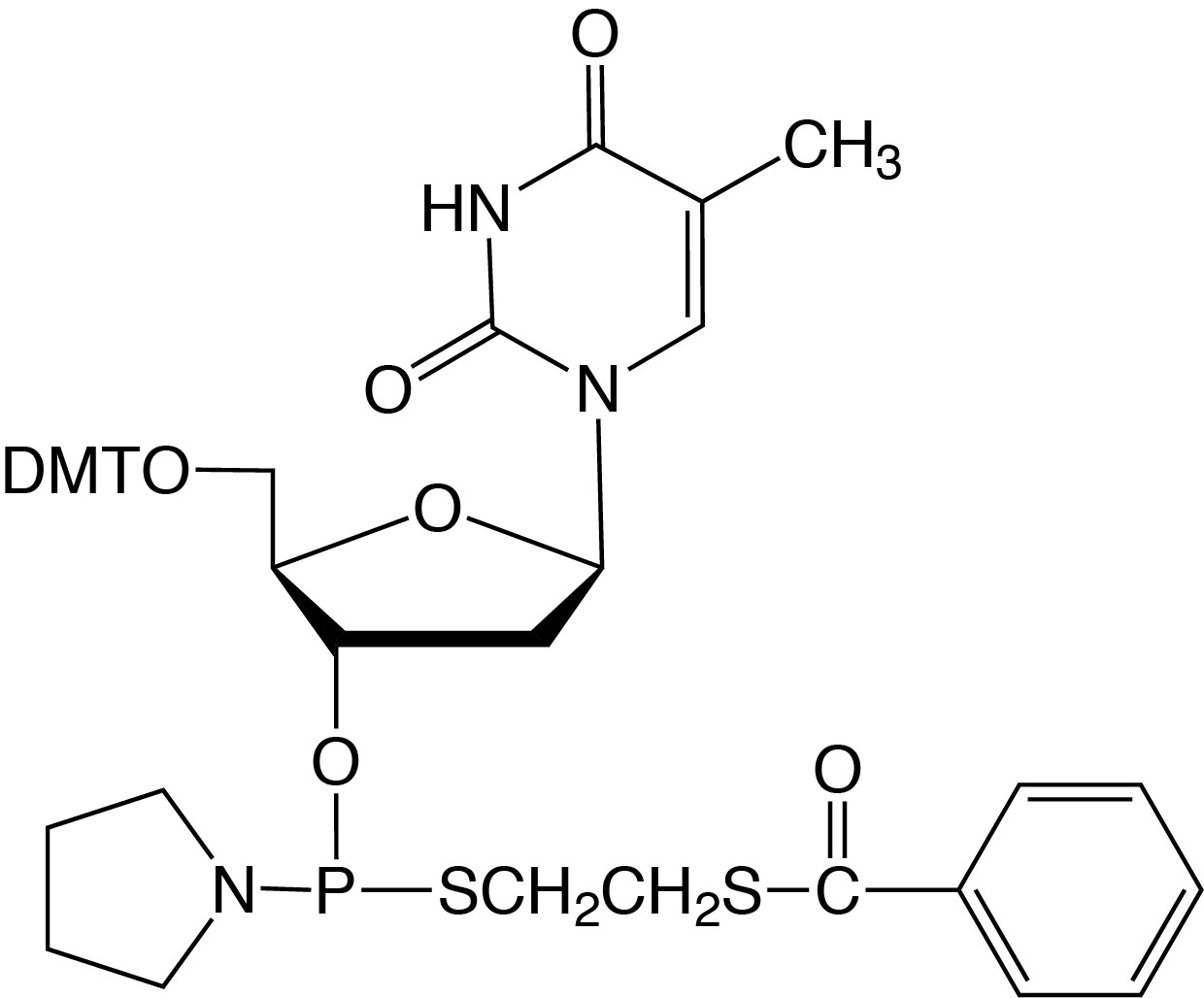
Description: dT-Thiophosphoramidite
5'-Dimethoxytrityl-2'-deoxyThymidine,
3'-[(Β-thiobenzoylethyl)-(1-pyrrolidinyl)]-thiophosphoramidite |
| Formula: C44H48N3O8PS2 |
M.W.: 841.97 |
F.W.: 336.32(dithioate) |
Diluent: 10% (v/v) Anhydrous Dichloromethane in Anhydrous Acetonitrile |
| Coupling: See Technical Bulletin |
| Deprotection: See Technical Bulletin for details (Technical Bulletin). |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: No data available |
THIOPHOSPHORAMIDITES
Replacing two non-bridging oxygen atoms with sulfur atoms in a DNA phosphodiester linkage creates a phosphorodithioate (PS2) linkage.1 Like natural DNA, the phosphorodithioate linkage is achiral at phosphorus. This analog is completely resistant to nuclease degradation and forms complexes with DNA and RNA with somewhat reduced stabilities.2 Moreover, it has been found that PS2-ODNs bind proteins with a higher affinity than their phosphodiester analogues2-6 suggesting that PS2-ODNs may have additional utility in the form of sulfur-modified phosphate ester aptamers (thioaptamers)3,6-8 for therapeutic and diagnostic applications. Thiophosphoramidites are now commercially available after recent work at AM Biotechnologies (www.thioaptamer.com).
- Thiophosphoramidites (ThioPAs) are not soluble in anhydrous acetonitrile diluent. Rather, 10% DCM (v/v) in acetonitrile is an ideal diluent for all four of the thioPAs for a final amidite concentration of 0.15 M.
- ThioPAs are somewhat less stable than normal DNA phosphoramidites in anhydrous acetonitrile containing 10% DCM; however, the coupling efficiency of all four thioPAs is not reduced after two days in solution at room temperature.
- After synthesis, the thioPA bottle on the synthesizer should be replaced with one containing acetonitrile diluent and the synthesizer line flushed with acetonitrile.
A typical cycle for the solid-phase synthesis of a PS2 linkage is different from a standard cycle for the synthesis of normal phosphate linkages. After coupling, the resulting thiophosphite triester is then sulfurized with DDTT. Capping is carried out AFTER sulfurization.
Upon completion of the automated synthesis, deprotection is carried out using a concentrated ammonia:ethanol (3:1, v:v) mix containing 20 mM DTT at 55 °C for 15-16 h.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200