3'-Propargyl-5-Me-dC CPG
Catalog Number: 20-2982-xx
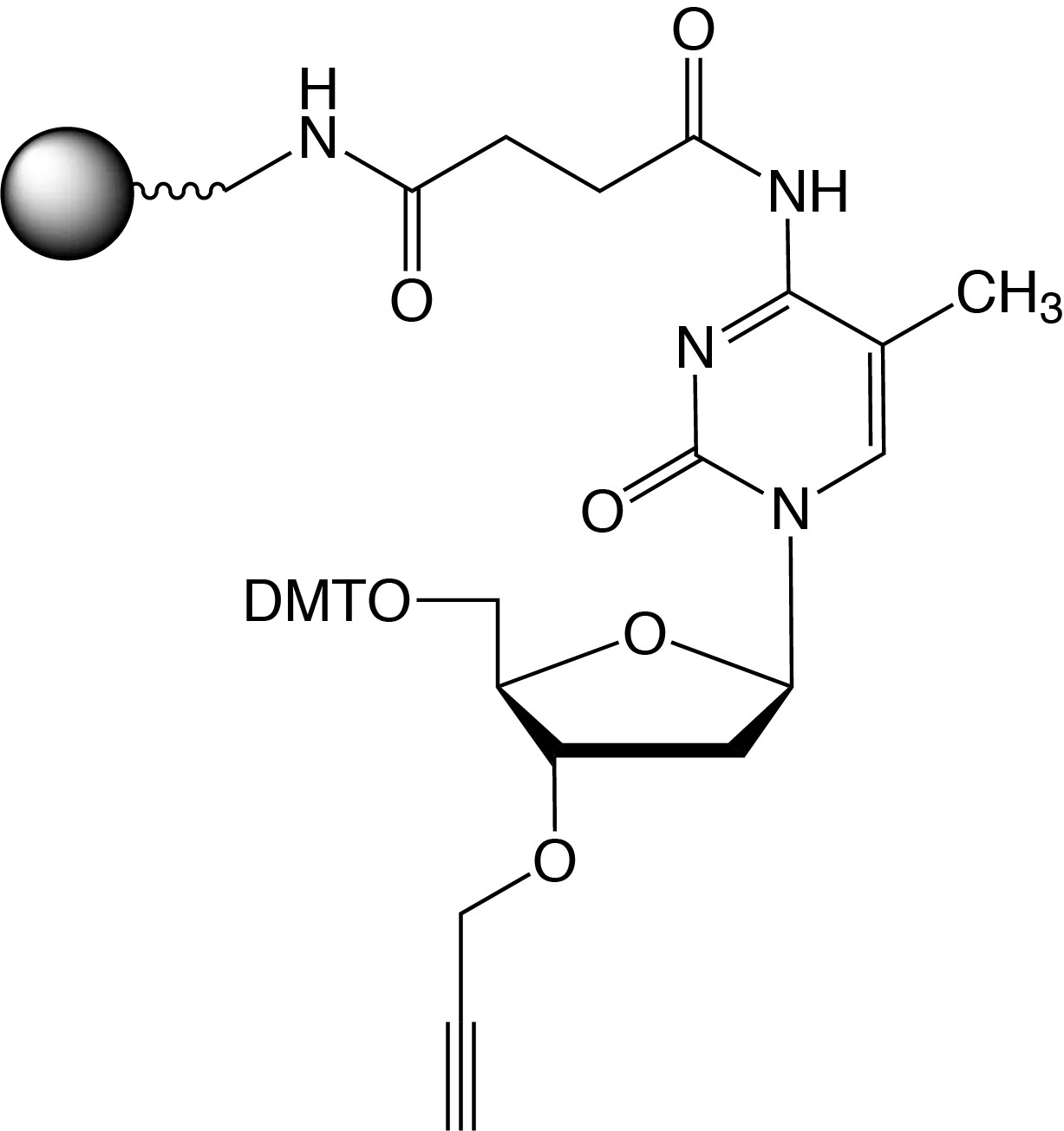
Description: 3'-Propargyl-5-Me-dC CPG
| 5'-Dimethoxytrityl-3'-propargyl-N-succinoyl-long chain alkylamino-CPG, 5-methyl-2'-deoxyCytosine |
|
|
F.W.: 341.26 |
|
| Coupling: No changes needed from standard method recommended by synthesizer manufacturer. |
| Deprotection: Cleavage of the oligonucleotide from this support requires 2 hours at room temperature with ammonium hydroxide. Complete the deprotection as required by the nucleobases. |
| Storage: Controlled room temperature or lower, dry |
| Stability in Solution: |
BIOLOGICAL MIMICS
Our product, 5’-I-dT-CE Phosphoramidite, has been moved to the click chemistry section of this WEB site and and 2,4-Difluorotoluene (F), as a non-polar mimic of thymidine, has been discontinued.
|
|
CLICK DNA AND RNA LIGATION
Ligation of an oligo containing a 5’-azide with an oligo containing a 3’-propargyl group using Click Chemistry leads to a triazole linkage that has been shown to have in vivo biocompatibility. This technique has been used to synthesize DNA constructs up to 300 bases in length. When the resultant triazole linkage was placed in a PCR template, various polymerases were able to copy the sequence correctly. The linkage has also been shown to be compatible with transcription and rolling circle amplification, as well as gene expression in E. coli. In the RNA world, a hammerhead ribozyme containing the triazole linkage at the substrate cleavage site has been shown to retain its activity. A large variety of applications is envisaged for this biocompatible chemical ligation. Support for this technology is offered with the help of Tom Brown’s group at the University of Southampton.
|
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200