Catalog Number: 50-1905-xx
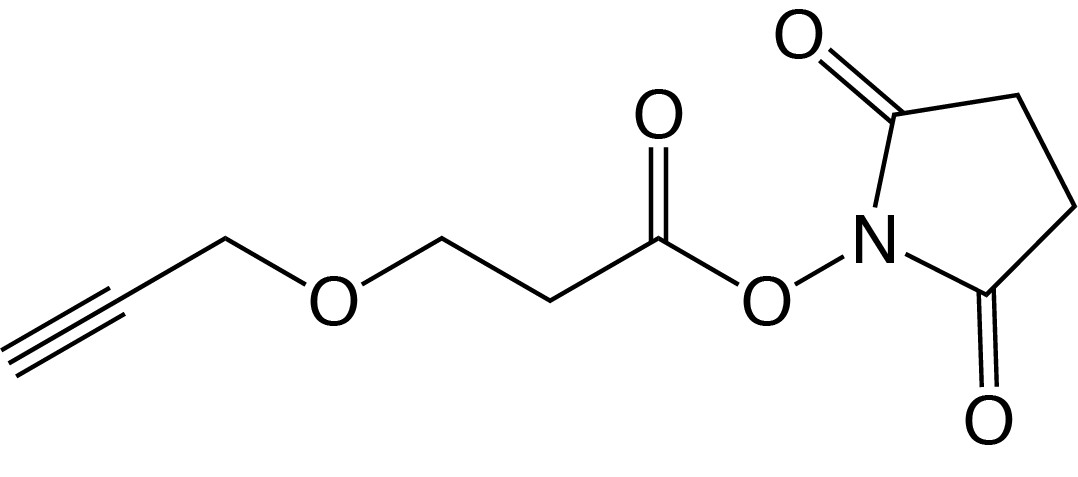
Description: Alkyne-NHS Ester
| 3-propargyloxypropanoic acid N-hydroxysuccinimidyl ester |
| Formula: C10H11NO5 |
M.W.: 225.20 |
F.W.: 110.11 |
Diluent: Anhydrous Acetonitrile
2.3mg/60uL, 23mg/0.60mL
|
| Labeling: For labeling 0.2µmoles of an amino-modified oligonucleotide, dissolve 2.3mg of the Alkyne-Modifier NHS Ester in 60µL of Acetonitrile (DMSO or DMF are also acceptable). Dissolve the amino-modified oligonucleotide in 500µL 0.1 M Carbonate/Bicarbonate buffer (pH 9). Add 12µL of the Alkyne-Modifier NHS Ester solution and vortex. Let react for 2 hours at room temperature and desalt. |
| Deprotection: Not Applicable |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: |
Oligonucleotides prepared using 5’-Hexynyl Phosphoramidite are stable to standard deprotection conditions and exhibit a slightly increased retention time on RP HPLC. Azides are not compatible with oligonucleotide synthesis using phosphoramidites so a post-synthesis reaction is required. Azidobutyrate NHS Ester is used1 for azido-modification of amines at either the 3’-end or the 5’-end of an oligo and it can even be used for internal modification on an Amino-Modifier-C6 dX residue within the sequence. Specific to the 5’-terminus, 5’-Bromohexyl Phosphoramidite is added in the last cycle. This modifier can then be easily transformed into a 5’-azido group by displacement of bromide using sodium azide.2 Alkyne NHS ester allows the functionalization of an amino moiety in a variety of molecules, including DNA and RNA oligonucleotides as well as peptides or proteins. We also offer a synthesis support for labelling the 3’ terminus of oligonucleotides with an alkyne group for use in Click Chemistry. This builds upon our 1,3-diol product portfolio with the serinol backbone. A 5’-iodo-modified oligonucleotide (prepared using 5’-Iodo-dT) can be quantitatively converted to the corresponding 5’-azide.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200