Catalog Number: 20-2040-xx
(20-2190-xx)
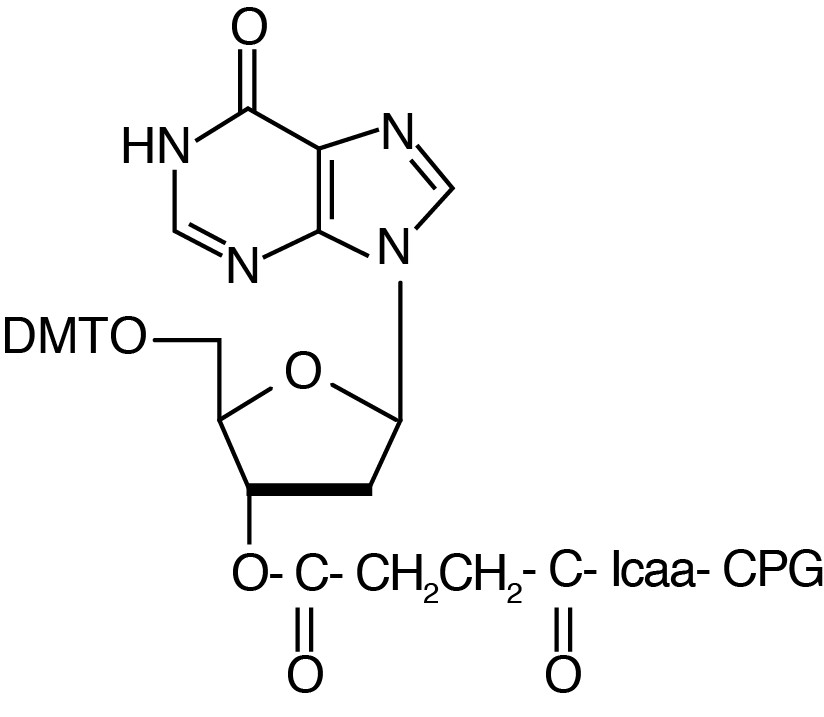
Description: dI-CPG 500
| 5'-Dimethoxytrityl-2'-deoxyInosine,3'-succinoyl-long chain alkylamino-CPG 500 |
| |
|
F.W.: 314.19 |
Diluent: Not Applicable |
| Coupling: This support should be used in a manner identical to normal protected nucleoside support since it contains the DMT group. |
| Deprotection: Deprotect using the protocol required by the nucleobases. |
| Storage: Controlled room temperature or lower, dry |
| Stability in Solution: Not Applicable |
duplex effects
The design of primers is frequently complicated by the degeneracy of the genetic code. Three strategies are now available to confront this problem. In the first, a mixed base addition (N) is used to form the degenerate site. This approach is best if the number of degenerate sites is small. A second option is the use of 2’-deoxyInosine or 2’-deoxyNebularine which exhibit low, but unequal, hydrogen bonding to the other four bases. The third option is the use of a universal nucleoside. In this strategy, the base analog does not hybridize significantly to the other four bases and makes up some of the duplex destabilization by acting as an intercalating agent. 3-Nitropyrrole 2’-deoxynucleoside (M) is the first example of a set of universal bases. Subsequently, 5-nitroindole was determined to be an effective universal base and to be superior to 3-nitropyrrole, based on duplex melting experiments.
The modified bases designated P and K show considerable promise as degenerate bases. The pyrimidine derivative P, when introduced into oligonucleotides, base pairs with either A or G, while the purine derivative K base pairs with either C or T. A dP dK mix also can serve as a mixed base with much less degeneracy than dA dC dG dT (N).
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200