Arabinonucleosides are epimers of ribonucleosides with the chiral switch being at the 2' position of the sugar residue. 2'-F-ANA adopts a more DNA-like β-type helix conformation, not through the typical C2'-endo conformation but, rather, through an unusual O4'-endo (east) pucker. However, the presence of the electronegative fluorine leads to a still significant increase (DTm1.2°C/mod) in melting temperature per modification.1 2'-F-ANA-containing oligonucleotides exhibit very high binding specificity to their targets. Indeed, a single mismatch in a 2'-F-ANA - RNA duplex leads to a DTm of -7.2°C and in a 2'-F-ANA - DNA duplex a DTm of -3.9°C.2
The presence of fluorine at the 2' position in 2'-F-ANA leads to increased stability to hydrolysis under basic conditions relative to RNA and even 2'-F-RNA.1,3 The stability of 2'-F-ANA to nucleases also makes this a useful modification for enhancing the stability of oligonucleotides in biological environments.2 2'-F-ANA hybridizes strongly to target RNA and, unlike most 2' modifications, induces cleavage of the target by RNase H. Phosphorothioate (PS) 2'-F-ANA is routinely used in these applications due to its increased nuclease resistance. Alternating 2'-F-ANA and DNA units provide among the highest potency RNase H-activating oligomers. Both the "altimer" and "gapmer" strand architectures consistently outperform PS-DNA and DNA/RNA gapmers.4
siRNA oligos were found to tolerate the presence of 2'-F-ANA linkages very well. High potency gene silencing was demonstrated5 with siRNA chimeras containing 2'-F-RNA and/or LNA and 2'-F-ANA. The high efficacy of these chimeras was attributed to the combination of the rigid RNA-like properties of 2'-F-RNA and LNA with the DNA-like properties of 2'-F-ANA.
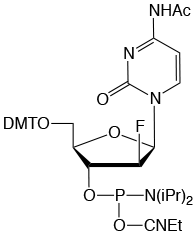
Formula: C41H49FN5O8P
M.W.: 789.83
F.W.: 307.17
Diluent: Anhydrous Acetonitrile
Coupling: 6 minute coupling time recommended.
Deprotection: No changes needed from standard method recommended by synthesizer manufacturer.
Storage: Storage: Refrigerated storage, maximum of 2-8°C, dry
Stability in Solution: 1-2 days
Please Note: 2'-F-ANA is covered by intellectual property. Key patents covering siRNA and antisense applications are as follows: WO/2009/146556 (siRNA); WO 03064441 and WO 0220773 (antisense).
- E . Viazovkina, M.M. Mangos, M.I. Elzagheid, and M.J. Damha, Curr Protoc Nucleic Acid Chem, 2002, Chapter 4, Unit 4 15.
- J.K. Watts, and M.J. Damha, Can. J. Chem., 2008, 86, 641-656.
- J.K. Watts, A. Katolik, J. Viladoms, and M.J. Damha, Org Biomol Chem, 2009, 7, 1904-10.
- A. Kalota, et al., Nucleic Acids Res., 2006, 34, 451.
- G.F. Deleavey, et al., Nucleic Acids Res., 2010, 38, 4547-4557, J.K. Watts, et al., Nucleic Acids Res., 2007, 35, 1441-1451, T. Dowler, et al., Nucleic Acids Res., 2006, 34, 1669-1675.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200