Cell Culture Contamination Control: What Are Mycoplasma?
The Stealthy Invader
What Are Mycoplasma and Why Are They so Feared?
Mar 26, 2024 | Informative Articles

Featured in this article:
• What is mycoplasma contamination?
• What happens when a cell culture is contaminated by mycoplasma?
In the world of microbiology, there exist tiny organisms causing big trouble – mycoplasma.
Mycoplasma are the smallest prokaryotic organisms and the most common contaminants in cell cultures. They can easily pass through sterile filtering systems and are resistant to commonly used antibiotics. Mycoplasma contamination is a serious problem for academic and biopharmaceutical laboratories due to its impact on cell properties and safety of biological products. A contamination often remains undetected and pose significant challenges to cell culture experiments, potentially comprising data integrity and research outcomes. It is estimated that mycoplasma are present in almost every lab and that about 5 to 30 % of the world’s cell lines are contaminated with mycoplasma, causing enormous costs and effort [1].
In this article we give a brief overview of mycoplasma contamination. Learn about the biology of mycoplasma and the consequences of a mycoplasma contamination to understand how cells are contaminated.
What is Mycoplasma Contamination? The Biology of Mycoplasma
Mycoplasma Structure
Mycoplasma are the smallest self-replicating bacteria (about 0.15 - 0.3 µm) and belong to the class of mollicute [1]. In fact, mycoplasma and mollicutes are commonly used as synonyms. Unlike other bacteria they lack a cell wall, makes them capable of assuming various shapes and resistant to commonly used antibiotics. Due to their flexible membrane and small size mycoplasma are able to pass through filtering systems.
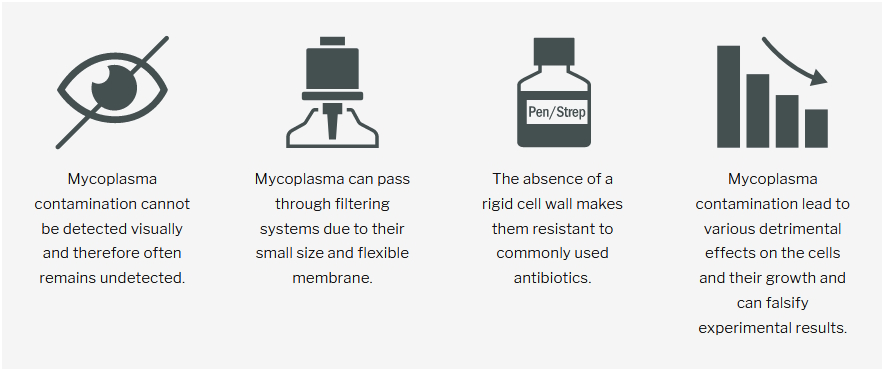
They cannot be detected visually - neither by the naked eye nor by microscope. Even highly contaminated cell cultures do not show any turbidity or other obvious symptoms. 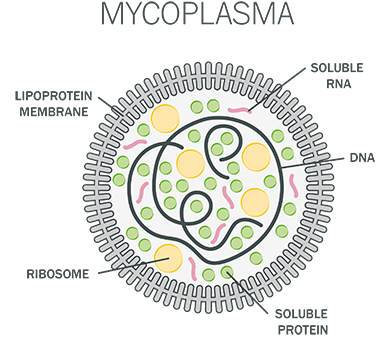
What makes mycoplasma so special?
• Absence of cell wall
• Flexible membrane
• Invisible
• Pass through filters
• Resistant to common antibiotics
Mycoplasma lack a cell wall, making them resistant to commonly used
antibiotics. The flexible membrane allowing them to shrink to diameters
< 0.45 µm and pass through filtering systems.
What happens when a cell culture is contaminated by mycoplasma? Consequences of a mycoplasma contamination
Mycoplasma species are known to infect a variety of eukaryotic cells, including human, animal and plant cells.
When a cell culture becomes infected with mycoplasma, it can lead to various detrimental effects on the cells and
their growth. Mycoplasma, being simple parasitic organisms, rely on the
host cells for their metabolic needs. Initially, they attach to the host
cell and then can merge with its membrane. Following this, mycoplasma
replicate rapidly, overgrowing significantly the host cells.
Unlike other bacterial contaminants, mycoplasma
contamination doesn't typically cause visible changes in the growth
medium. However, its impact on cell cultures can be profound and can
lead to:
• Altered cell metabolism
• Inhibition of cell growth and proliferation
• Reduced transfection efficiency
• Changes in gene and protein expression
• Chromosomal abnormalities
• Impair nucleic acid synthesis
• Apoptosis

These effects can vary depending on the cell
line and the species of mycoplasma involved. Nevertheless, mycoplasma
contamination poses a serious challenge to cell culture research, as it
can compromise the reliability, reproducibility and validity of
experimental results.
The presence of mycoplasma in cell culture has different side effects, including:
• Loss of time
• Loss of money
• Loss of valuable cells
• Potential dissemination of inaccurate findings through publications
• Misleading publications
• Biosafety concerns
If a contamination remains undetected,
contaminated cultures can endanger scientific data, delay research
projects and jeopardise the safety of biopharmaceutical products.
Therefore, it's crucial for researchers to be vigilant in detecting and
preventing mycoplasma contamination in cell cultures.
The impact of mycoplasma contamination in cell culture can be profound
and represent a serious problem for academic and biopharmaceutical
laboratories due to its impact on cell properties and safety of
biological products.
References
[1] Nikfarjam L, Farzaneh P. Prevention and detection of Mycoplasma contamination in cell
culture. Cell J. 2012 Winter;13(4):203-12. Epub 2011 Dec 22. PMID: 23508237; PMCID:
PMC3584481.