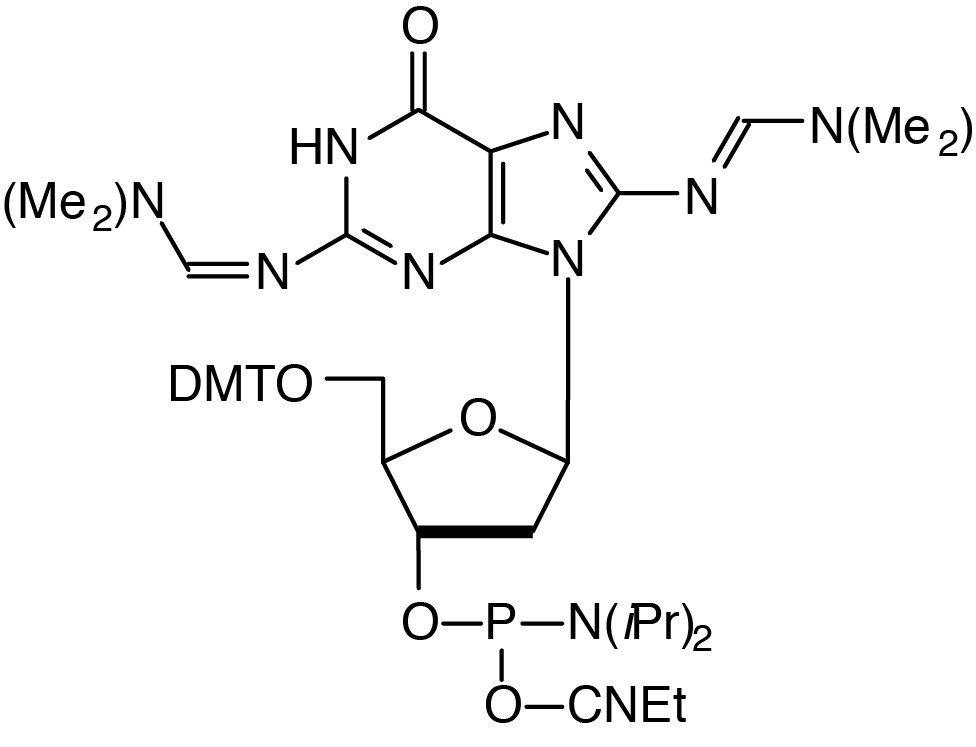
| 8-Amino-dG-CE Phosphoramidite |
Catalog Number: 10-1079-xx
Description: 8-Amino-dG-CE Phosphoramidite
5'-Dimethoxytrityl-N2,N8-bis(dimethylaminomethylidine)-8-amino-2'-deoxyGuanosine,
3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C46H59N10O7P |
M.W.: 895.01 |
F.W.: 344.22 |
Diluent: Anhydrous Acetonitrile |
| Coupling: No changes needed from standard method recommended by synthesizer manufacturer. |
| Deprotection: Cleave and deprotect with ammonium hydroxide containing 0.1M 2-mercaptoethanol, (7µl/ml) at 55° for 20 hours. |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 2-3 days |
8-Amino-dA and 8-amino-dG are useful in triplex formation due to the presence of the additional amino groups.
2’-DeoxyXanthosine (dX) is a naturally occurring nucleoside that may be derived from oxidative deamination of 2’-deoxyGuanosine (dG). dX has a similar bonding pattern to thymidine and it may base pair with dA, with such purine-purine interactions causing duplex distortion. dX also featured in attempts to extend the genetic alphabet with a new base pair of dX and pyrimidine-2,4-diamine nucleoside. dX has also interested researchers in the field of DNA damage and repair since it is a product of nitric oxide-induced mutagenesis.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200