| CAG Trimer Phosphoramidite |
Catalog Number: 13-1102-xx
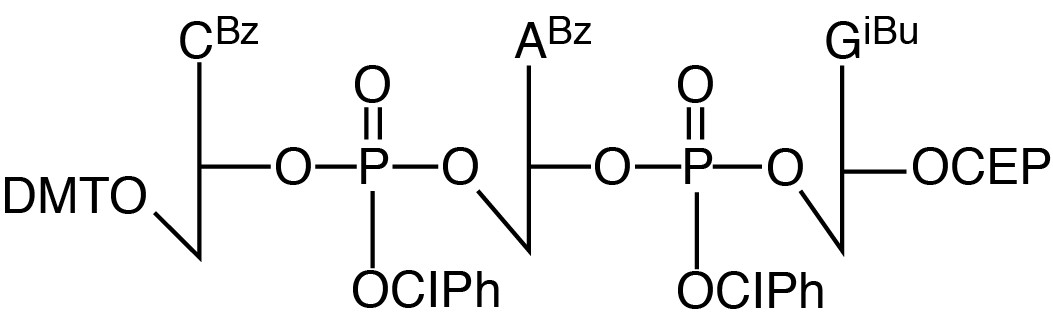
Description: CAG Trimer Phosphoramidite
5'-Dimethoxytrityl-N4-benzoyl-2'-deoxyCytidine-3'->5'-o-chlorophenyl-phosphoryl-
N6-benzoyl-2'-deoxyAdenosine-3'->5'-o-chlorophenyl-phosphoryl-N2-isobutyryl-
2'-deoxyGuanosine-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C89H92Cl2N15O21P3 |
M.W.: 1869.5 |
|
Diluent: Anhydrous Acetonitrile/Dichloromethane 1:3 (v/v) |
| Coupling:15 minute coupling time recommended. |
| Deprotection: 30% NH4OH for 17 hours at room temperature followed by an additional 4 hours at 55°C. |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: 2-3 days |
Trimer Phosphoramidites
In principle, the simplest approach for oligonucleotide-directed mutagenesis would be the use of trimer phosphoramidites. Of the 64 possible combinations of codons, only 20 codons would be required to cover the 20 amino acids, although, in practice, several codons will likely be duplicated depending on the organism. Our trimers use the protection scheme described1-3 by Kayushin et al. There is a concern that the sequence of the trimers has to be verified. For example, CAT coding for histidine, has to be differentiated from TAC, coding for tyrosine. These two trimers have virtually identical lipophilicity and their identity cannot be clearly confirmed by HPLC. This problem has been solved4 using HPLC electrospray mass spectrometric analysis of the trimers, which provides data confirming molecular weight and sequence.
In Table 1, the trimers, their coding amino acid and their reaction factor (RF) are listed. The reaction factor is critical since the trimers will likely be mixed and they have differing reactivity in the coupling reaction. RF for AAC is 1.0 and for TAC is 1.6. Therefore, 1.6 equivalents of TAC are needed for every 1.0 equivalent of AAC for equal coupling. Mixtures can easily be made using equimolar solutions or the molecular weight of each trimer has to be used to generate the appropriate weights of each trimer to use if mixing by weight. An example of the preparation of a mixture of all 20 trimers is shown in the right column of Table 1 and completed in the footnotes.
All of the trimers are now available individually so that researchers can prepare custom mixtures. A mixture of all 20 trimers designed to produce equal coupling of all 20 is also available. If you require custom production of a specific mixture, please e-mail support@glenresearch.com for a quotation and projected delivery.
Example of Preparation of Trimer Mixture
Prepare 530 mg of the trimer mix, taking the amount (mg) for each trimer from the right column. Dissolve the trimer mix in dichloromethane (highest grade possible; acid-free). Evaporate to dryness to produce a homogenous mixture of all 20 trimers.
Example of Preparation of Trimer Mixture for the Synthesizer
Dissolve 530 mg, which is equivalent to 20X10 µmoles (normalized for RF) of the trimer mix in 2.0 mL of acetonitrile-dichloromethane mixture, 1:3 v/v to produce a 0.10N solution of trimers, ready for use in a synthesizer.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200