DBCO-dT-CE Phosphoramidite
Catalog Number: 10-1539-xx
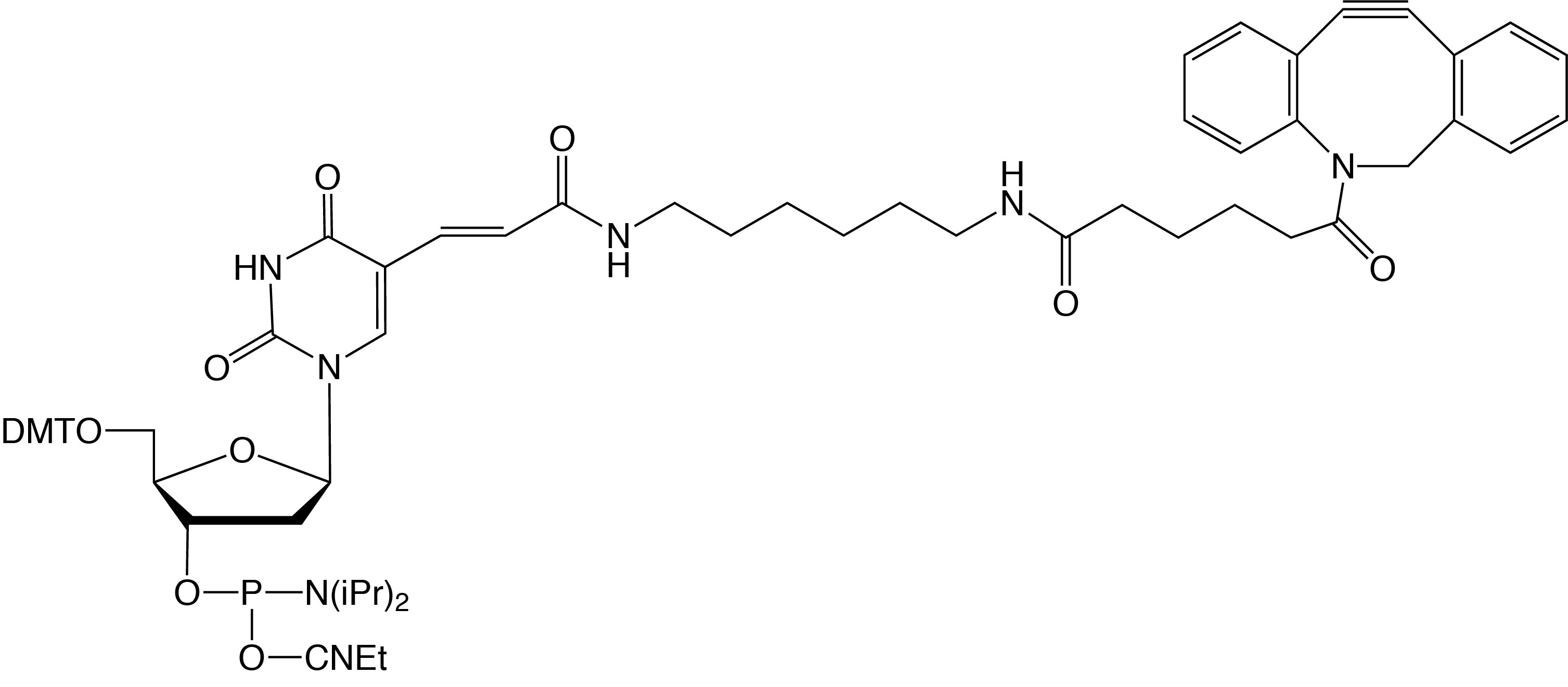
Description: DBCO-dT-CE Phosphoramidite
5'-Dimethoxytrityl-5-[(6-oxo-6-(dibenzo[b,f]azacyclooct-4-yn-1-yl)-
capramido-N-hex-6-yl)-3-acrylimido]-2'-deoxyUridine,
3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C69H80N7O11P |
M.W.: 1214.57 |
F.W.: 773.77 |
Diluent: Anhydrous Acetonitrile/Dichloromethane 1:3 (v/v) - code :40-4450-20
Please request with your order if required.
|
| Coupling: 12 minute coupling time is recommended. |
| Deprotection: As required by nucleobases. Compatible with 30% ammonium hydroxide for 2 hours at 65 °C or 17 hours at room temperature. AMA (ammonium hydroxide/40% methylamine 1:1) for 2 hours at room temperature will give acceptable results. |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: 1-2 days |
COPPER-FREE CLICK CHEMISTRY
At Glen Research, our goal was to offer a copper-free click phosphoramidite reagent with the following properties:
- Simple to use
- Stable in solution on the synthesizer
- Stable to ammonium hydroxide and AMA
- Excellent click performance in 17 hours or less at room temperature
From the variety of cyclooctyne-based copper-free click reagents so far described, we have chosen to offer compounds based on a dibenzo-cyclooctyne (DBCO) structure. We are offering 5’-DBCO-TEG Phosphoramidite for preparing oligos with a 5’-DBCO modification and DBCO-dT-CE Phosphoramidite for inserting a DBCO group at any position within the oligonucleotide. DBCO-sulfo-NHS Ester is also offered for post-synthesis conjugation reactions. DBCO-modified oligos may be conjugated with azides in organic solvents, such as DMSO, or aqeous buffers. Depending on the azide used, the reaction will go to completion in 4-17 hours at room temperature. Simple desalting on a Glen Gel-Pak™ leads to a product with virtually quantitative conjugation efficiency.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200