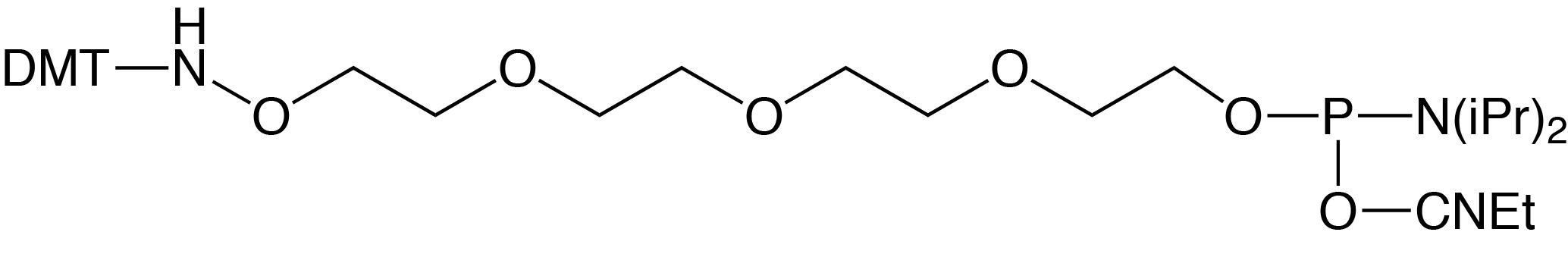
5'-Aminooxy-Modifier-11-CE Phosphoramidite
Catalog Number: 10-1919-xx
Description: 5'-Aminooxy-Modifier-11-CE Phosphoramidite
10-[N-Dimethoxytrityl-aminooxyethyl)]-triethyleneglycol-1-[(2-cyanoethyl)-
(N,N-diisopropyl)]-phosphoramidite |
| Formula: C38H54N3O8P |
M.W.: 711.82 |
F.W.: 271.21 |
Diluent: Anhydrous Acetonitrile
Add fresh diluent to product vial to recommended concentration and swirl vial occasionally over several minutes until product is completely dissolved. (Some oils may require between 5 and 10 minutes.) Use care to maintain anhydrous conditions. In case of transfer to alternate vial type, ensure recipient vial has been pre-dried. For more information, see http://www.glenres.com/ProductFiles/Technical/TB_ABITransfer.pdf.
|
| Coupling: 3 minute coupling time recommended. |
| Deprotection: Deprotect using the protocol required by the nucleobases. Compatible with standard deprotection conditions. See Technical Bulletin for details regarding oxime formation (Technical Bulletin). |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: 2-3 days |
The disulfide thiol modifier may be used for introducing 3'- or 5'-thiol linkages. Dithiol Phosphoramidite (DTPA) is a disulfide-containing modifier designed to functionalize synthetic DNA or RNA with multiple thiol groups and can be incorporated at any position of the oligonucleotide. Each DTPA addition leads to two thiol groups. This modifier was designed for optimal tethering of oligonucleotides to a gold surface but it can also be used for multiple reactions with maleimides and other thiol-specific derivatives. 5'-Carboxy-Modifier C10 is a unique linker designed to be added at the terminus of an oligonucleotide synthesis. It generates an activated carboxylic acid N-hydroxysuccinimide (NHS) ester suitable for immediate conjugation on the synthesis column with molecules containing a primary amine, resulting in a stable amide linkage. PC Amino-Modifier is a photocleavable C6 amino-modifier, part of our line of photocleavable (PC) modifiers.
5'-AminoOxy-Modifier 11 is based on a tetraethylene glycol linkage for improved solubility and for reducing the potential negative impact on hybridization of the oligo. The oxime formed from the reaction of alkyloxyamines with aldehydes creates a stable covalent bond. In comparison, the imine formed by the conjugation of primary amines with aldehydes is not stable to acidic or basic conditions and requires subsequent reduction with borohydride to form stable amine conjugates. 5'-Maleimide Modifier Phosphoramidite, developed at the University of Barcelona, incorporates a maleimide cycloadduct that is stable to ammonium hydroxide at room temperature. This phosphoramidite can be incorporated into DNA and RNA with both phosphate and phosphorothioate linkages. A retro–Diels-Alder reaction deprotects the maleimide immediately prior to conjugation.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200