DNA Methylation
One of the fastest growing fields in biology and cancer research is epigenetics. While the underlying genetic code defines which proteins and gene products are synthesized, it is epigenetic control that defines when and where they are expressed. This dynamic control of gene expression is essential for X chromosome inactivation, embryogenesis, cellular differentiation and appears integral to memory formation and synaptic plasticity.
In 2009, two reports1,2 described the discovery of 5-hydroxymethyl-2'-deoxyCytidine (hmdC), a novel dC modification in Purkinje neurons and embryonic stem cells. Later, a third report found this modification to be strongly enriched in brain tissues associated with higher cognitive functions.3 This dC modification is generated by the action of α-ketoglutarate dependent ten eleven translocation (TET) enzymes, which oxidizes 5-Me-dC to hmdC. This finding stimulated discussion about active demethylation pathways that could occur, e.g., via base excision repair (BER), with the help of specialized DNA glycosylases. Alternatively, one could envision a process in which the hydroxymethyl group of hmdC is further oxidized to 5-formyl-dC (fdC) or 5-carboxy-dC (cadC) followed by elimination of either formic acid or carbon dioxide4,5. Glen Research has supported this research since its inception by providing the building blocks for the synthesis of oligonucleotides containing all the new dC derivatives - hmdC, fdC and cadC. The first generation hmdC phosphoramidite was fairly very well accepted but requires fairly harsh deprotection conditions. Therefore, a second generation building block (5-Hydroxymethyl-dC II) developed by Carell and co-workers that is compatible with UltraMild deprotection was introduced.6 5-Formy-dC III has been designed to meet all of the requirements to prepare an oligo containing all of the methylated variants.
- LI>S. Kriaucionis, and N. Heintz, Science, 2009, 324, 929-30.
- M. Tahiliani, et al., Science, 2009, 324, 930-935.
- M. Münzel, et al., Angewandte Chemie-International Edition, 2010, 49, 5375-5377.
- D. Globisch, et al., PLoS One, 2010, 5, e15367.
- S.C. Wu, and Y. Zhang, Nat Rev Mol Cell Biol, 2010, 11, 607-20.
- M. Münzel, D. Globisch, C. Trindler, and T. Carell, Org Lett, 2010, 12, 5671-3.
- A.S. Schroder, et al., Angewandte Chemie-International Edition, 2014, 53, 315-318.
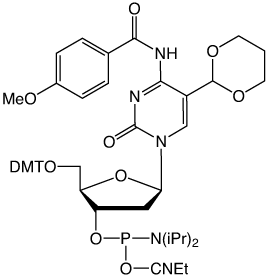
M.W.: 950.02
F.W.: 317.19 FW: 375.27 (acetal)
Diluent: Anhydrous Acetonitrile
Coupling: 3 minute coupling time is recommended.
Deprotection: In 30% ammonium hydroxide, use dmf-dG during synthesis and deprotect for 17 hours at room temperature. If deprotection in sodium hydroxide is required for use in oligos containing other epigenetic bases, use ibu-dG and Ac-dC during synthesis and deprotect using 0.4 M NaOH in MeOH/water 4:1 (v/v) for 17 hours at room temperature. Removal of acetal protecting group: if sodium hydroxide was used during deprotection, desalt the oligo and dry down, otherwise, dry down directly. Take up oligo in 80% acetic acid in water and let react for 6 hours at 20°C
Storage: Storage: Freezer storage, -10 to -30°C, dry
Stability in Solution: 1-2 days
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200