Beta L-DNA is the mirror image version of naturally occurring D-DNA.
L-DNA and D-DNA share identical structures that differ only in terms of
stereochemistry and generally have identical physical and chemical
properties. The difference in their stereochemistry results in
differences in their interactions with chiral molecules, D-DNA will only
bind to its D-DNA complement to form right-handed helices, and
likewise, L-DNA will only bind to its L-DNA complement to form
left-handed helices. For this reason, enzymes that interact with D-DNA,
including nucleases, typically won’t interact with L-DNA. The unique
properties of L-DNAs have made them attractive for many biological
applications such as Aptamers, Molecular Beacons, Molecular Tagging, and
Drug Nanocarriers. Note that the procedure for synthesizing L-DNA
oligonucleotides is very similar to that of D-DNA oligonucleotides.
Please see GR31.2 for more details.
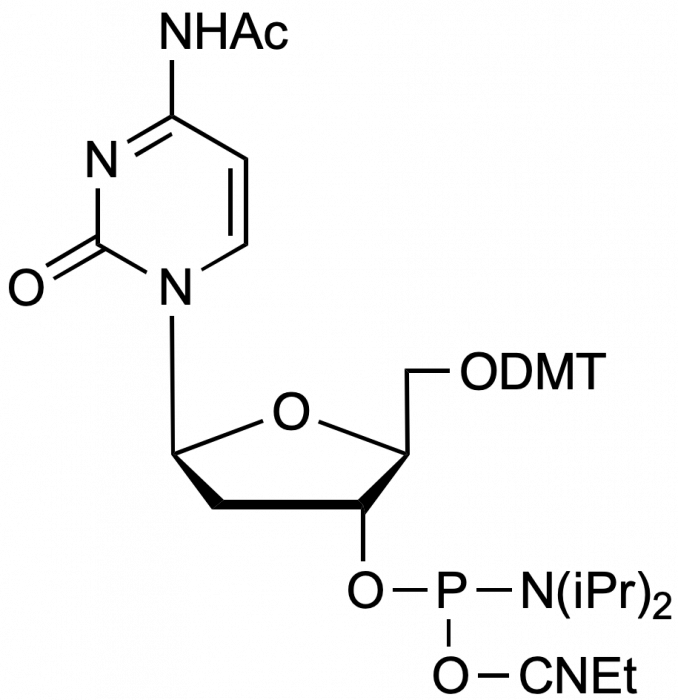
beta-L-Ac-dC-CE Phosphoramidite
5'-Dimethoxytrityl-N-acetyl-beta-L-2'-deoxyCytidine,3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
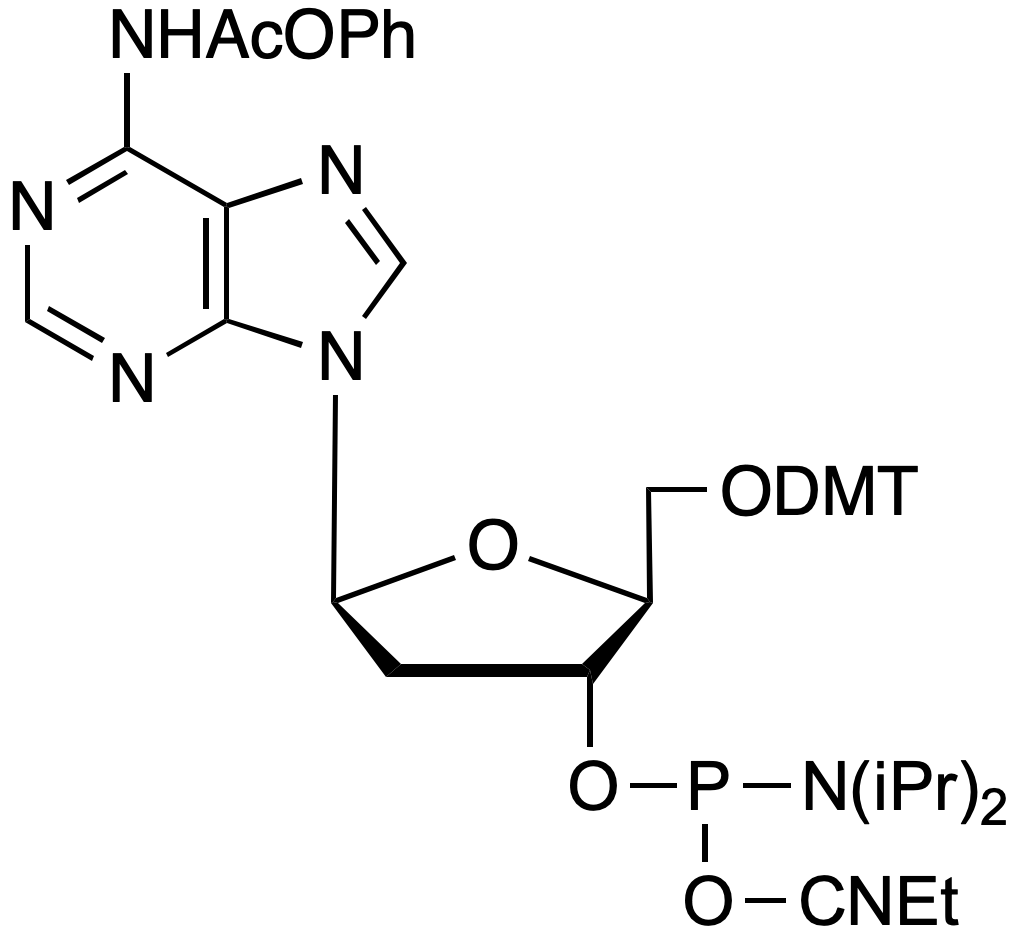
beta-L-Pac-dA-CE Phosphoramidite
5'-Dimethoxytrityl-N-phenoxyacetyl-beta-L-2'-deoxyAdenosine,3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
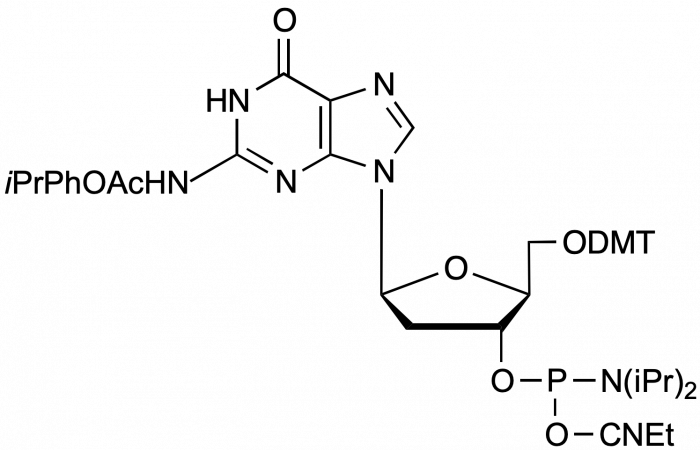
beta-L-iPr-Pac-dG-CE Phosphoramidite
5'-Dimethoxytrityl-N-p-isopropyl-phenoxyacetyl-beta-L-2'-deoxyGuanosine,3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
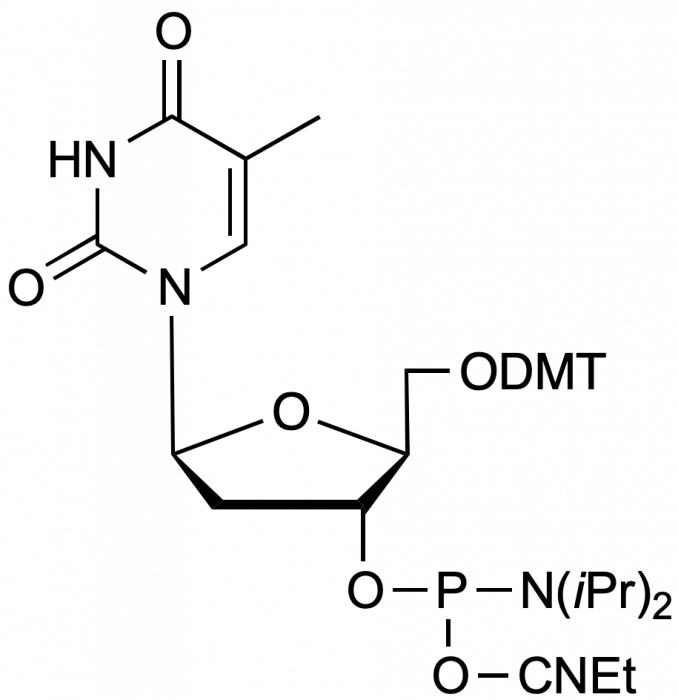
beta-L-dT-CE Phosphoramidite
5'-Dimethoxytrityl-beta-L-2'-deoxyThymidine,3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200