Mutagenicity, cytotoxicity and endocrine disruptor and chromosomal aberration tests
Mutagenicity Kits - Identify mutagenic compounds
This section contains reagents and kits manufactured by Xenometrix used for identifying a wide variety of chemicals and products for their genotoxic/mutagenic effects using Ames microfluctuation technology. These kits include those such as Ames II, Ames MPF PENTA 1 and Ames MPF PENTA 2, Ames MPF 1535. The range now also includes the new miniaturised Ames MicroAmes6 98/100.
_test.jpg)
Xenometrix's standard tests are available as complete kits ( excluding plasticware). They are standardised and quality controlled, designed to run in in a liquid format on 384 well plates. The assay system is a microsystems listed in the ICH M7 guideline and it is a version of the Ames fluctuation test cited in the OECD / FDA guidelines. Several publications showing a high concordance with the agar plate method are available. Xenometrix also offers all accessories to perform the Ames assay, such as S9, positive controls, S9 Cofactor solution, etc.
Endocrine Disruptors - In vitro Estrogen and Androgen Activity
Another product range is the “XenoScreen YES/YAS” and “Xenoscreen XL YES YAS” for the detection of substances activating or inhibiting human estrogen and androgen receptors, via agonistic or antagonistic action.
Cytotoxicity Assays
Single and multiple endpoint cytotoxicity assays covering a wide range of mechanisms and read-outs. The test kits are typically used in the early screening phase to determine cytotoxicity endpoints in eukaryotic cells. Xenometrix is the only company offering 4-endpoint cytotoxicity assays.
In vitro Micronucleus Test, Chromosomal Aberration Tests
Complete FISH chromosomal aberaation testing in under an hour- the fastest FISH on the market!
Using specially designed FISH probes and a set of carefully optimized solutions allow the detection of centromeres and telomeres in human cells to a very high degree of sensitivity, in both ex vivo and cell lines, in as fast as 1 hour.
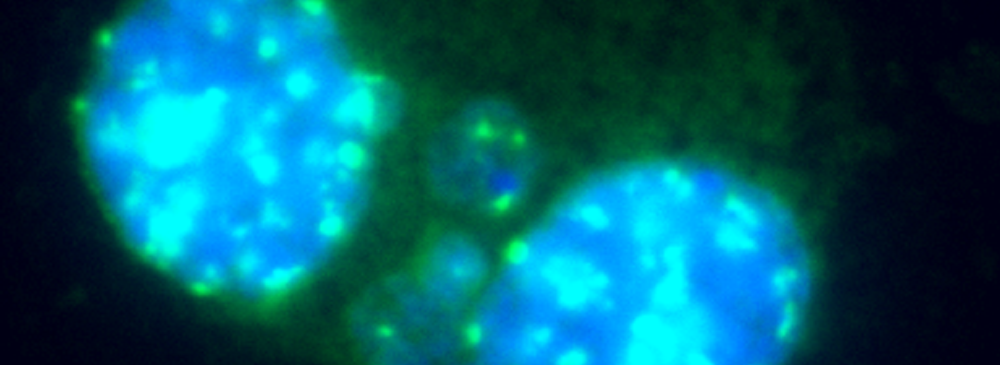
In Vitro MicroNucleus Test MNT (OECD TG487) – Principle
The in vitro MicroNucleus test is one of
the most successful and reliable assays for the detection of chemicals
with genotoxic potential. Chemicals or physical agents may generate
acentric chromosomes (clastogen event) or inappropriately segregate
whole chromosomes (aneugen event) leading to genetic damage in the form
of an erratic, smaller nucleus called micronucleus.
Both in vitro and
in vivo studies can be designed. The former is generally performed
using mammalian cell lines, typically upon administration of test items
(chemicals or mixtures). In vivo micronucleus assay is performed on
peripheral blood cells for cytogenetics research and investigations on
the genotoxic effects of radiation and irradiation.
Click here for the product page