| 1-Me-A-CE Phosphoramidite |
Catalog Number: 10-3501-xx
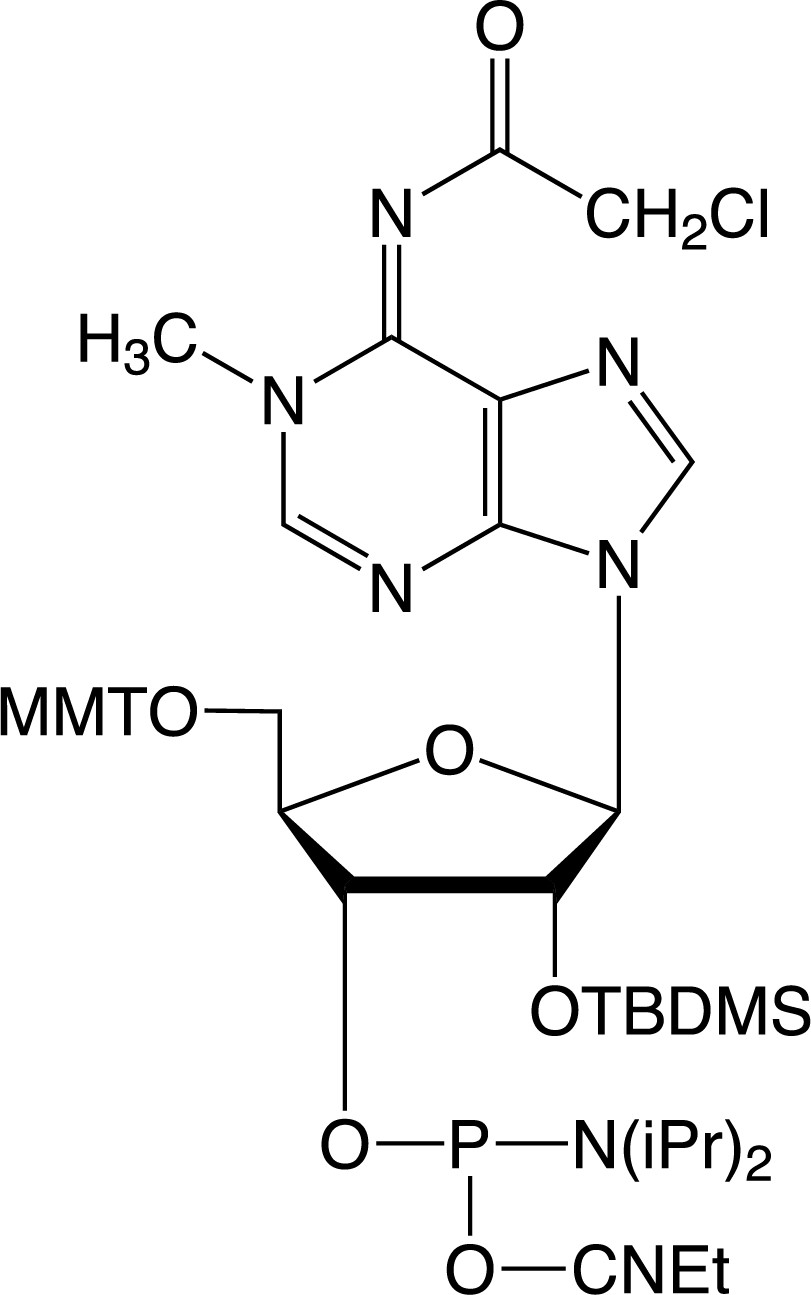
Description: 1-Me-A-CE Phosphoramidite
5'-Monomethoxytrityl-N6-chloroacetyl-1-methyl-Adenosine, 2'-O-TBDMS-
3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C48H63ClN7O7PSi |
M.W.: 944.57 |
F.W.: 344.24 |
Diluent: Anhydrous Acetonitrile |
| Coupling: 15 minute coupling time recommended. Monomers that allow for UltraMILD deprotection are recommended. (Pac-A:10-3000-xx, Ac-C: 10-3015-xx, iPr-Pac-G: 10-3021-xx, U: 10-3030-xx ). |
| Deprotection: 2 M Ammonia in methanol, 24 hours at Room Temperature using UltraMILD amidites or 60 hours at Room Temperature if using amidites with standard protecting groups. |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: Similar to dA,C,G,T-CE Phosphoramidites |
Methylation of adenosine at position 1 produces a drastic functional change in the nucleobase. 1-Methyladenosine (pKa 8.25) is a much stronger base than adenosine (pKa 3.5). N-1 methylation excludes participation of the adenine base in canonical Watson–Crick base pairing and provides a positive charge to the nucleobase. This modification also alters the hydrophobicity of the base, the stacking properties, the ordering of water molecules and the chelation properties. The base may become involved in non-canonical hydrogen bonding, in electrostatic interactions and, in general, it may contribute to the conformational dynamics of the tRNA.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200