| 2-F-dI-CE Phosphoramidite |
Catalog Number: 10-1082-xx
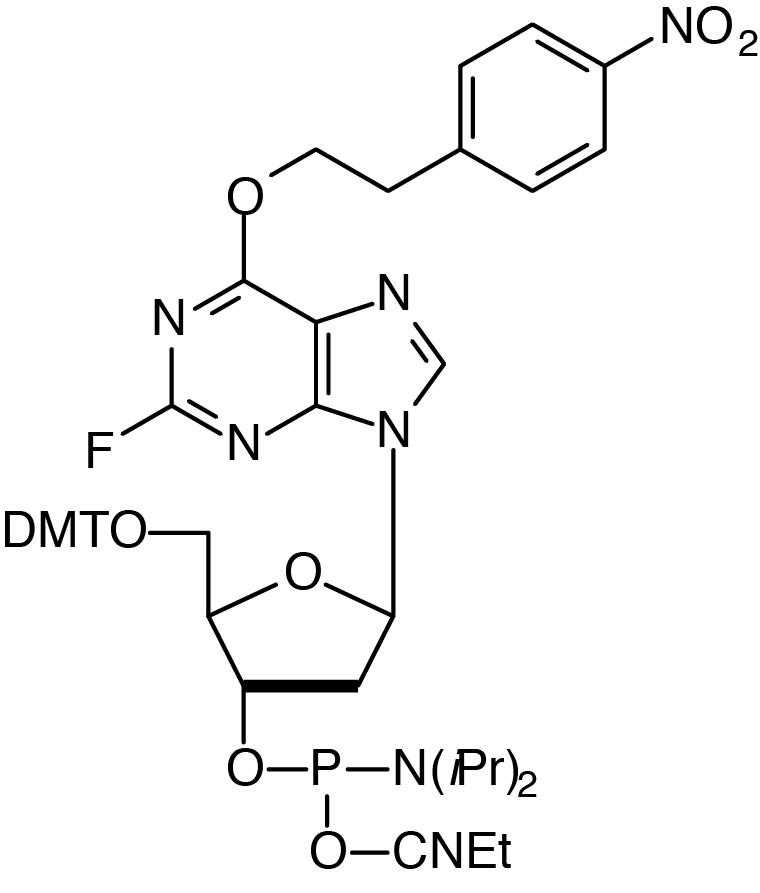
Description: 2-F-dI-CE Phosphoramidite
5'-Dimethoxytrityl-2-fluoro-O6-p-nitrophenylethyl-2'-deoxyInosine,3'-
[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C48H53FN7O9P |
M.W.: 921.96 |
F.W.: varies, 2F=332.18 |
Diluent: Anhydrous Acetonitrile |
| Coupling: No changes needed from standard method recommended by synthesizer manufacturer. |
| Deprotection: See Technical Bulletin for details (www.glenresearch.com/Technical/TB_2-F-dI.pdf). Technical Bulletin |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 2-3 days |
CONVERTIBLE NUCLEOSIDES
The convertible nucleoside strategy is one of the most versatile methods for producing modifications in bases to examine their effects on DNA structure and activity. In some cases, with versatility comes difficulty in that the convertible base is modified after oligonucleotide synthesis. The chemistry is sometimes complex and base composition analysis of the final oligonucleotide is required to verify structure. The convertible dU monomer can be used to introduce a variety of modifications at the convertible position, including N, O and S modifications. Convertible F-dC is by far the simplest approach to the preparation of oligonucleotides containing F-dC - normal ammonium hydroxide treatment effects the conversion to F-dC. Convertible dA has been used to prepare oligonucleotides containing multiple points for attachment to solid supports. In this way, high capacity affinity supports for the purification of DNA binding proteins have been prepared. 2-F-dI is a convertible nucleoside for the preparation of 2’-dG derivatives following the displacement of the 2-fluorine by primary amines.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200