| 2-Thio-dT-CE Phosphoramidite |
Catalog Number: 10-1036-xx
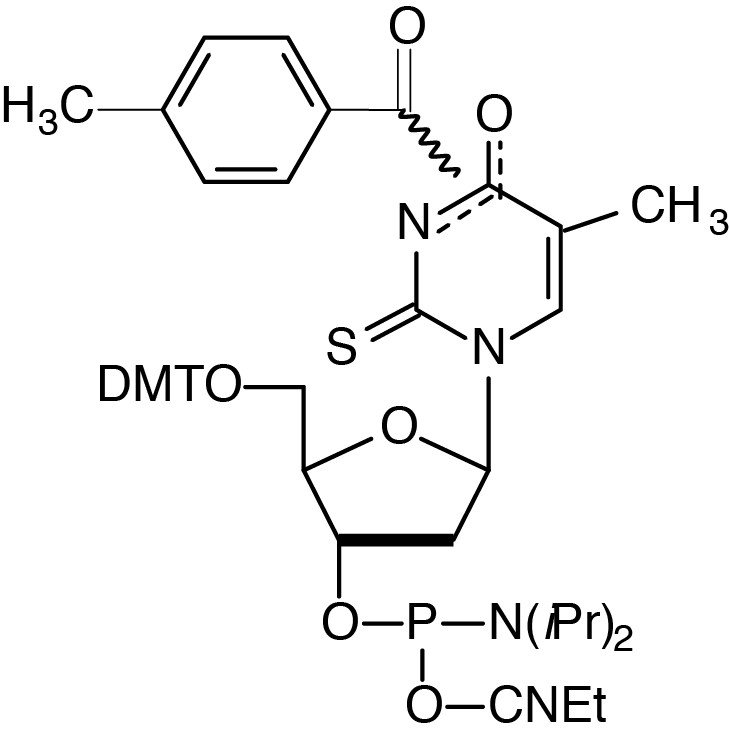
Description: 2-Thio-dT-CE Phosphoramidite
5'-Dimethoxytrityl-(N3/O4-toluoyl)-2'-deoxy-2-thioThymidine,
3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C48H55N4O8PS |
M.W.: 879.02 |
F.W.: 320.26 |
Diluent: Anhydrous Acetonitrile |
| Coupling: No changes needed from standard method recommended by synthesizer manufacturer. |
| Deprotection: No changes needed from standard method recommended by synthesizer manufacturer. Technical Bulletin |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 2-3 days |
The C-nucleoside 2’-deoxypseudouridine, in contrast to dU, forms stable C:pseudoU-A triplets. 2-Aminopurine lacks groups critical for hydrogen bonding and is a mildly fluorescent base.
Demand for sulfur modified bases continues to expand for investigations of oligonucleotide structure, but primarily for cross-linking purposes. 6-Thio-dG, 4-Thio-dT and 4-thio-dU are very useful modifications for photo cross-linking and photoaffinity labelling experiments. Oligos containing 2-thio-dT are useful in examining protein-DNA interaction by acting as photosensitizing probes. The thiocarbonyl group in 2-thio-dT is especially interesting in that it is available to react with compounds associating with the minor groove of DNA. 2-Amino-A forms a very stable base pair with T containing three hydrogen bonds but the stability of the base pair with 2-thio-T is greatly diminished. Due to steric interactions between the 2-thio group of thymidine and the 2-amino group of 2-amino-A, the base pair contains only a single hydrogen bond. Oligos containing 2-amino-dA and 2-thio-dT exhibit high affinity for natural oligonucleotides but show little affinity for other similar oligos even of a complementary sequence.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200