| 3'-PT-Amino-Modifier C6 CPG |
Catalog Number: 20-2956-xx
Description: 3'-PT-Amino-Modifier C6 CPG
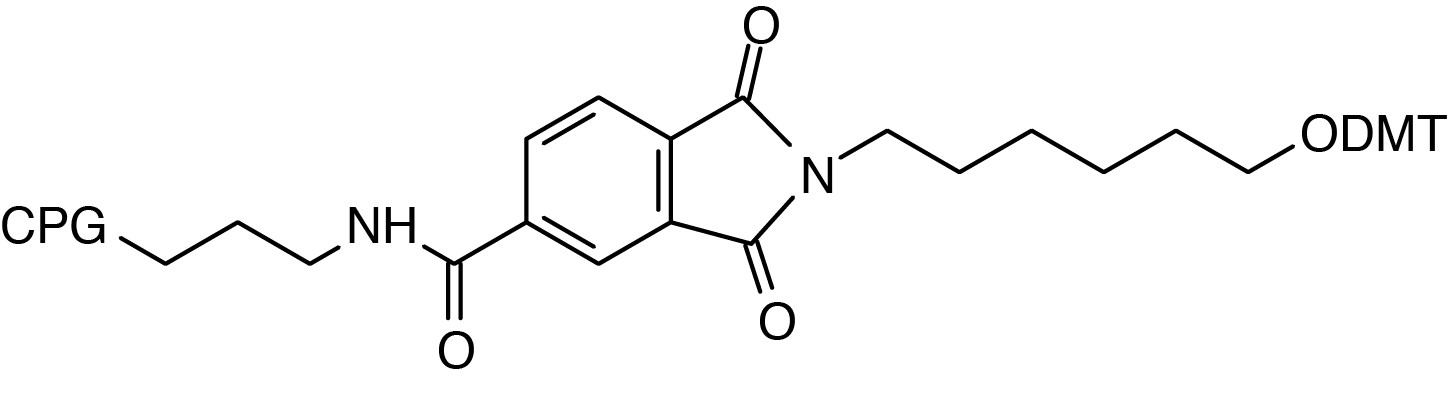
| N-(6-(O-Dimethoxytrityl)-hexyl)-(2-carboxamide)-phthalimidyl-lcaa-CPG |
|
|
F.W.:179.15 |
Diluent: Not Applicable |
| Coupling: This support should be used in a manner identical to normal protected nucleoside support since it contains the DMT group. |
| Deprotection: Cleavage of the oligonucleotide from this support using ammonium
hydroxide requires overnight at 55°C or 48 hours at room temperature; in
AMA, 10 minutes at 65°C or 2 hours at room temperature is required.
This treatment will complete the deprotection of standard nucleobases.
For additional cleavage conditions see Technical Bulletin. |
| Storage: Freezer Storage, -10 to -30°C, dry |
| Stability in Solution: Not Applicable |
3’-MODIFIERS
3'-Amino-Modifier CPGs, containing amino groups protected with the
base-labile Fmoc group, are designed to functionalize the 3'-terminus of
the target oligonucleotide by the introduction of a primary amine. In
an alternative approach, the nitrogen destined to become the 3'-amino
group is included in a phthalimide (PT) group which is attached to the
support through an amide group attached to the aromatic ring. This
simple linkage is very stable to all conditions of oligonucleotide
synthesis and contains no chiral center. Using an extended ammonium
hydroxide treatment (55°C for 17 hours), the cleavage of the amine from
the phthalimide is accomplished along with the deprotection of the
oligonucleotide. ABI-style columns are supplied unless otherwise
requested.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200