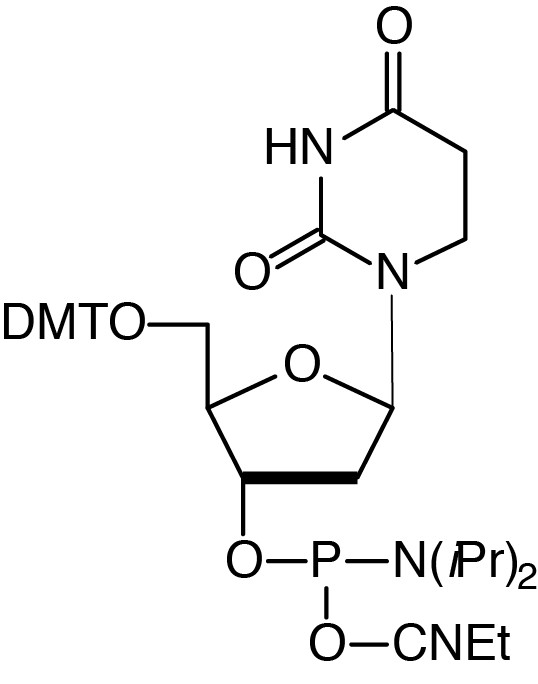
| 5,6-Dihydro-dU-CE Phosphoramidite |
Catalog Number: 10-1550-xx
Description: 5,6-Dihydro-dU-CE Phosphoramidite
5'-Dimethoxytrityl-5,6-dihydro-2'-deoxyUridine),3'-[(2-cyanoethyl)-
(N,N-diisopropyl)]-phosphoramidite |
| Formula: C39H49N4O8P |
M.W.: 732.81 |
F.W.: 292.19 |
Diluent: Anhydrous Acetonitrile |
| Coupling: Monomers that allow for UltraMILD deprotection must be used. (dA:10-1601-xx,dC: 10-1015-xx, dG: 10-1621-xx, dT: 10-1030-xx ). To avoid any exchange of the iPr-Pac group on the dG with acetyl, use the UltraMild Cap Mix A (40-4210-xx/ 40-4212-xx). |
| Deprotection: UltraMILD deprotection: 0.05M Potassium Carbonate in Methanol, 4 hours at Room Temperature or 2 hours at room temperature in Ammonium Hydroxide. |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 2-3 days |
DNA DAMAGE/REPAIR
Cellular DNA is constantly being damaged by oxidation and alkylation, by free radicals, and by ultraviolet and ionizing radiation. The body has therefore evolved a number of repair enzyme systems to excise and repair these lesions. The 8-oxo purine monomers allow investigation of the structure and activity of oligonucleotides containing an 8-oxo mutation which is formed naturally when DNA is subjected to oxidative conditions or ionizing radiation. 5,6-Dihydro pyrimidines are naturally occurring compounds that are structural components of alanine transfer RNA. Dihydrouracil and the hydroxy pyrimidines are major base damage products formed by exposure of DNA to ionizing radiation.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200