| 5'-Amino-dT-CE Phosphoramidite |
Catalog Number: 10-1932-xx
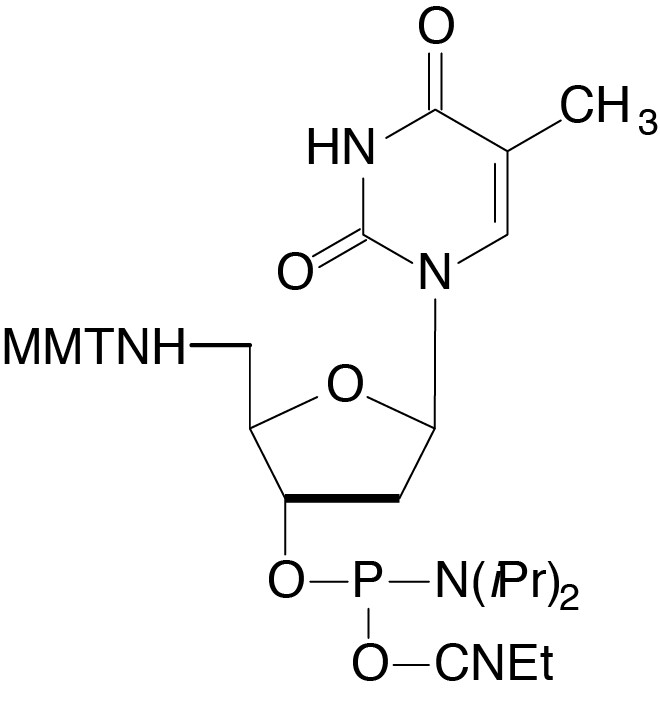
Description: 5'-Amino-dT-CE Phosphoramidite
5'-monomethoxytritylamino-2'-deoxyThymidine,3'-[(2-cyanoethyl)-
(N,N-diisopropyl)]-phosphoramidite |
| Formula: C39H48N5O6P |
M.W.: 713.81 |
F.W.: 303.21 |
Diluent: Anhydrous Acetonitrile |
| Coupling: No changes needed from standard method recommended by synthesizer manufacturer. |
| Deprotection: Deprotect as required by nucleobases.WARNING: As with other trityl-protected amines, drying down the oligo after cleavage and deprotection without addition of an non-volatile base (for example, TRIS) will lead to loss of the Trityl protecting group. See Technical Bulletin for details (Technical Bulletin). |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 24 hours |
CHAIN TERMINATORS
In situations where ligation must be blocked at the 5’ terminus, 5’-OMe-dT may be used. 5’-OMe modification of a strand of siRNA using 5’-OMe-T can control guide strand selection and targeting specificity.15’-Amino-dT terminates an oligonucleotide with a 5’-amino group which may be used for attaching a peptide or a PNA sequence. To avoid polymerase extension at the 3’ terminus, 2’,3’-dideoxynucleoside and 3’-deoxynucleoside CPGs have proved to be effective. 2’,3’- Phosphoramidites are designed to be used with the 5’-phosphoramidites and supports. Since these phosphoramidites have no DMT group, they are not compatible with purification by the DMT-on technique. Ion exchange HPLC or PAGE should be used to purify these dideoxy terminated oligos to ensure that shorter sequences (containing 3’-OH) groups are removed. (3’-Termination can also be effected using a 3’-3’ linkage formed using 5’-supports, or 3’-spacer C3 CPG.)
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200