| Abasic II Phosphoramidite |
Catalog Number: 10-1927-xx
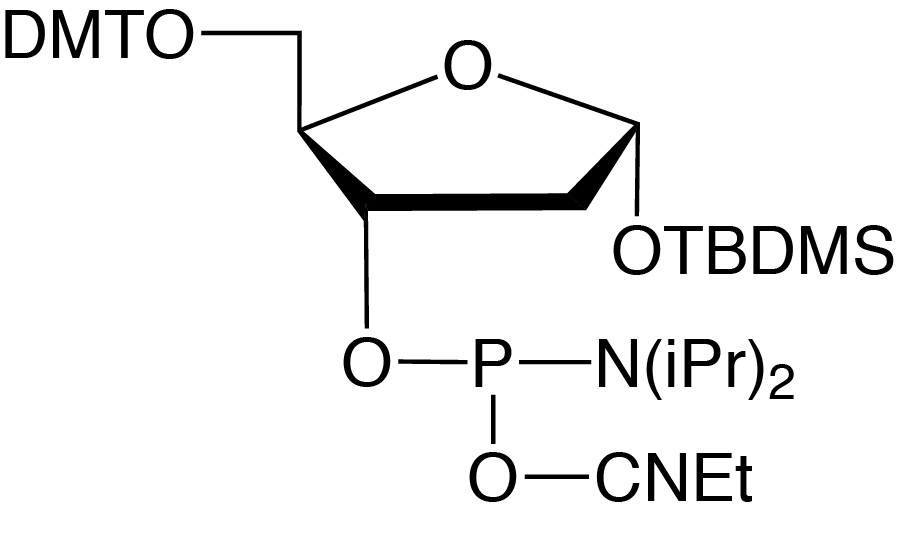
Description: Abasic II Phosphoramidite
5-O-Dimethoxytrityl-1-O-tert-butyldimethylsilyl-2-deoxyribose-
3-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite |
| Formula: C41H59N2O7PSi |
M.W.: 750.98 |
F.W.: 196.1 |
Diluent: Anhydrous Acetonitrile
Add fresh diluent to product vial to recommended concentration and swirl vial occasionally over several minutes until product is completely dissolved. (Some oils may require between 5 and 10 minutes.) Use care to maintain anhydrous conditions. In case of transfer to alternate vial type, ensure recipient vial has been pre-dried. For more information, see http://www.glenres.com/Technical/TB_ABITransfer.pdf.
|
| Coupling: 6 minute coupling time recommended. |
| Deprotection: Synthesize using acetyl-protected dC and deprotect in 30% Ammonium Hydroxide/40% Methylamine 1:1 (AMA) at 65°C for 10 min OR Synthesize using dmf-dG and deprotect in EtOH/30% Ammonium Hydroxide (1:3) for 24 hrs at room temperature. To generate the abasic site, dry the oligo down and take up in 0.25mL 80% Acetic acid. After 30 min at room temperature, add 0.25mL water and let sit for an additional 4 hrs. Quench with 0.5mL 2M TEAA and desalt on a Glen Gel-Pak™ column or equivalent. Store the oligo at pH 6-7 at 4°C or frozen. Do not dry; drying results in cleavage of the abasic site. See Technical Bulletin for details (Technical Bulletin). |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: 2-3 days |
8-Amino-G is formed along with 8-oxo-G as the major mutagenic lesions formed in DNA damage caused by 2-nitropropane. 2-Nitropropane is an industrial solvent and a component of paints, dyes and varnishes, and is also present in cigarette smoke. Thymine glycol (5,6-dihydroxy-5,6-dihydrothymine) is formed when thymine is subjected to oxidative stress, including ionizing radiation. Oxidation of the 5,6 double bond of Thymidine generates two chiral centers at C5 and C6. The cis-5R,6S form is generated as the predominant product along with the other diastereomer, the cis-5S,6R form. The presence of thymidine glycol in DNA has significant biological consequences and many organisms possess specific repair enzymes for the excision of this lesion. 2-Aminoimidazolone (Iz) and its hydrolysis product imidazolone (Z) are major oxidation products of G. Access to these two potential lesions is not possible during oligonucleotide synthesis because they are so base-labile. A suitable precursor, 8-methoxy-dG (8-OMe-dG), to dIz has now been described. The conversion of 8-OMe-dG to dIz takes place by irradiation of the oligonucleotide (1 mM) in 50 mM sodium cacodylate buffer, pH 7, in the presence of riboflavin (50 µM) for 2 minutes on a transilluminator (366 nm), under aerobic conditions at 4°C. Surprisingly for a photochemical reaction, the conversion is virtually quantitative.
Hydrolysis of nucleoside residues in DNA occurs to generate abasic sites. Most commonly, dA sites are hydrolyzed causing depurination and leading to abasic residues. For researchers trying to determine if their source of depurination in chemical synthesis of DNA is reagent, fluidics or protocol-based, we offer a depurination-resistant dA monomer. A new chemical method allows the generation of abasic sites in double and single stranded oligonucleotides using very mild specific conditions and with very low probability of side reactions. The original Abasic Phosphoramidite (10-1924) has been discontinued since it exhibits low coupling efficiency and the post-synthesis chemistry is fairly challenging. Abasic II Phosphoramidite1 is the replacement for the preparation of a true abasic site. This product has the advantage of simplicity in that the silyl group is removed post-synthesis using aqueous acetic acid. dSpacer has also been used successfully as a mimic of the highly base-labile abasic site.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200