| Chemical Phosphorylation Reagent II |
Catalog Number: 10-1901-xx
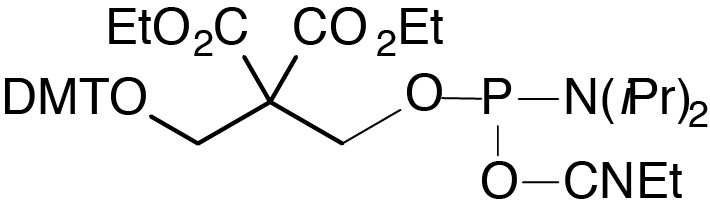
Description: Chemical Phosphorylation Reagent II
[3-(4,4'-Dimethoxytrityloxy)-2,2-dicarboxyethyl]propyl-(2-cyanoethyl)-
(N,N-diisopropyl)-phosphoramidite |
| Formula: C39H51N2O9P |
M.W.: 722.82 |
F.W.: 79.98 |
Diluent: Anhydrous Acetonitrile
Add fresh diluent to product vial to recommended concentration and swirl vial occasionally over several minutes until product is completely dissolved. (Some oils may require between 5 and 10 minutes.) Use care to maintain anhydrous conditions. In case of transfer to alternate vial type, ensure recipient vial has been pre-dried. For more information, see http://www.glenres.com/Technical/TB_ABITransfer.pdf.
|
| Coupling: 6 minute coupling time. Omit the capping step after the addition of this reagent! |
| Deprotection: If the final DMT is removed during synthesis, deprotection under standard conditions will eliminate the CPR II to yield a 5' Phosphate. If the DMT is retained, see Technical Bulletin for removal procedure (www.glenresearch.com/Technical/TB_CPR_II.pdf). Technical Bulletin |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 24 hours |
| Please Note: Chemical Phosphorylation Reagent II is covered by US Patent No.: 5,959,090 and European Patent: EP0816368 |
CHEMICAL PHOSPHORYLATION
Chemical Phosphorylation Reagent is most commonly used to phosphorylate the 5’-terminus of an oligonucleotide. Although this product is also successful in 3’-phosphorylation, 3’-Phosphate CPG allows direct preparation of oligonucleotides with a 3’-phosphate group. Chemical Phosphorylation Reagent II contains a DMT group on a side chain which is stable to base cleavage and can be left on the oligonucleotide for use in RP purification. The DMT group is later removed with aqueous acid and the side chain is eliminated after brief treatment with aqueous ammonium hydroxide to yield the 5’-phosphate.1 Solid CPR II is similar in performance to CPR II but it is easier to prepare aliquots since it is a powder. Many researchers treat synthesis supports with a hindered base (e.g., diethylamine, diisopropylethylamine, or DBU) post-synthesis to eliminate and remove the cyanoethyl phosphate groups. In this way, the acrylonitrile formed in situ is removed from the support and is not available to alkylate dT residues at the N3 position in the oligos. Since the sulfonylethyl group in 3’-Phosphate CPG is also susceptible to ß-elimination leading to oligo cleavage, this technique is not compatible with 3’-phosphate CPG. Using CPR II CPG, which is base labile but does not support ß-elimination, the cyanoethyl groups can be removed from the oligo prior to cleavage and base deprotection. ABI-style vials and columns are supplied unless otherwise requested.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200