Catalog Number: 10-5931-xx
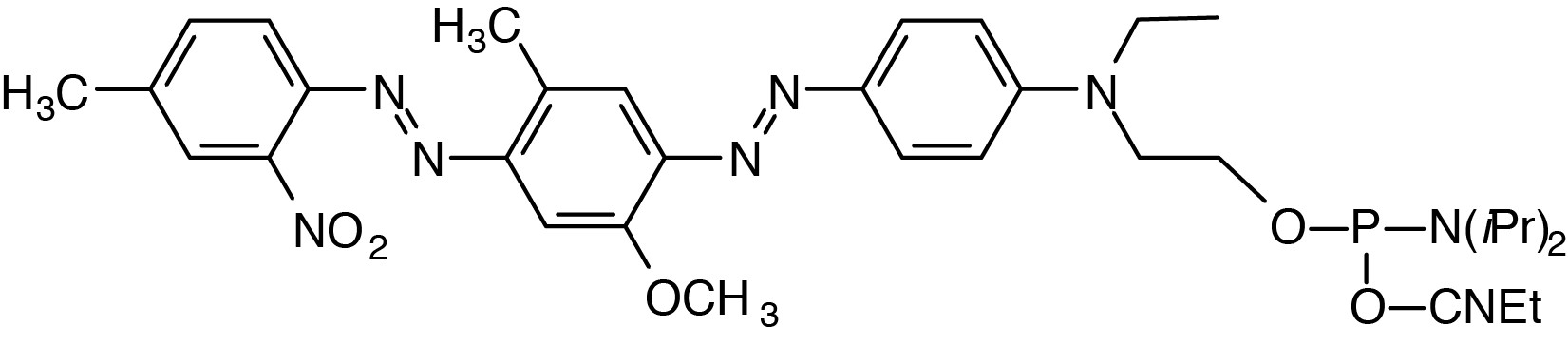
Description: 5'-BHQ-1 Phosphoramidite
4'-(2-Nitro-4-toluyldiazo)-2'-methoxy-5'-methyl-azobenzene-4''-(N-ethyl)-N-ethyl-
2-cyanoethyl-(N,N-diisopropyl)-phosphoramidite |
| Formula: C34H45N8O5P |
M.W.: 676.75 |
F.W.: 538.49 |
Diluent: Anhydrous Acetonitrile |
| Coupling: No changes needed from standard method recommended by synthesizer manufacturer. |
| Deprotection: No changes needed from standard method recommended by synthesizer manufacturer. |
| Storage: Freezer storage, -10 to -30°C, dry |
| Stability in Solution: 2-3 days |
| Please Note: 'Black Hole Quencher', 'BHQ-0', 'BHQ-1', 'BHQ-2' and 'BHQ-3' are trademarks of Biosearch Technologies, Inc., Novato, CA. The BHQ dye technology is the subject of pending patents and is licensed and sold under agreement with Biosearch Technologies, Inc.. Products incorporating the BHQ dye moiety are sold exclusively for R&D use by the end-user. They may not be used for clinical or diagnostic purposes and they may not be re-sold, distributed or re-packaged. |
Black Hole Quencher Dyes
With the growing popularity of red and near-infrared dyes, we are offering the Black Hole QuencherTM dyes (BHQs), whose physical properties are detailed in Table 1. BHQ dyes are robust dark quenchers that very nicely complement our existing product line. They are compatible with ammonium hydroxide deprotection, exhibit excellent coupling efficiencies, have large extinction coefficients and are completely non-fluorescent. Their absorbances are well-tuned to quench a variety of popular fluorophores – even those far into the red, such as Cy3 and Cy5. The dark quencher most typically used in a Molecular Beacon is Dabcyl. Because the quenching does not involve FRET, there is little, if any, dependence upon donor-acceptor spectral overlap. In a comprehensive paper by Marras, Kramer and Tyagi,1 the ability of BHQ-1 and BHQ-2 to quench 22 different fluorophores was evaluated. For shorter wavelength fluorophores such as fluorescein, the quenching efficiency was roughly the same as Dabcyl (91% – 93%). However, for dyes emitting in the far red, such as Cy5, the BHQ dyes were far superior – quenching the Cy5 with 96% efficiency, compared to 84% with Dabcyl. This may reflect the BHQ’s ability to form stable, non-fluorescent complexes which can be a plus even in FRET probes. Indeed, recent work suggests that these non-fluorescent complexes will form even in the absence of a hairpin stem structure used by Molecular Beacons.2
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200