Catalog Number: 10-1089-xx
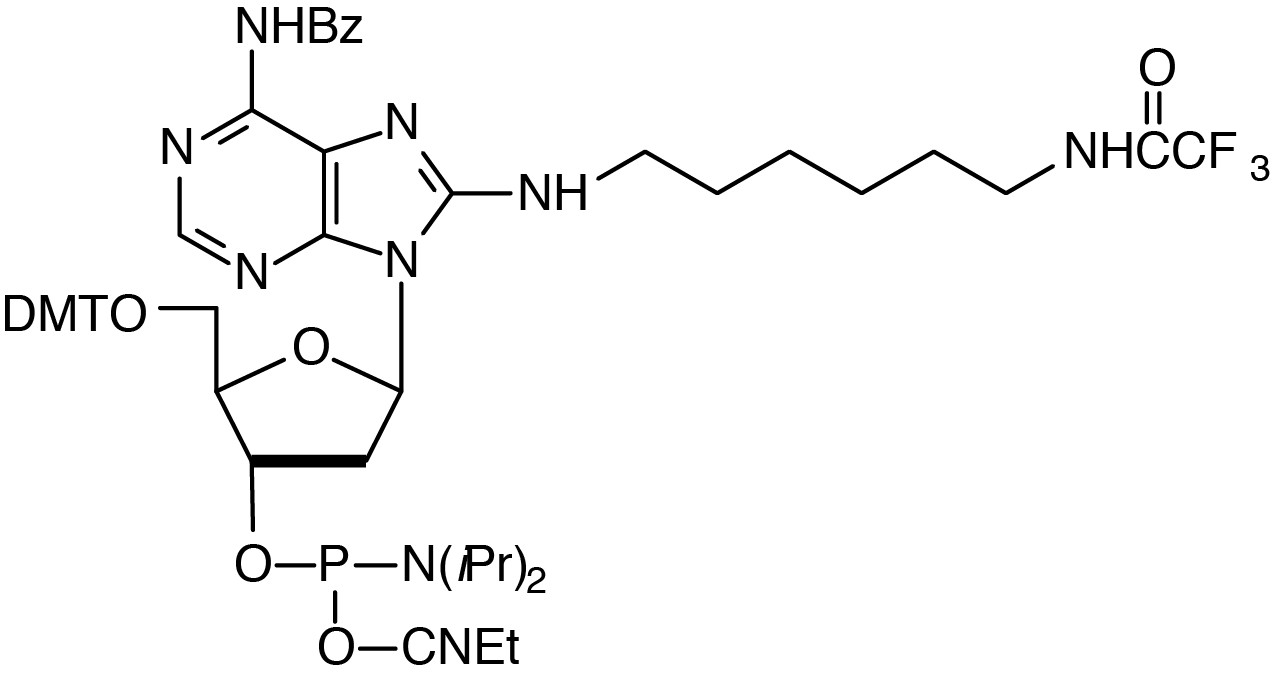
Description: Amino-Modifier C6 dA
5'-Dimethoxytrityl-N6-benzoyl-N8-[6-(trifluoroacetylamino)-hex-1-yl]-8-amino-
2'-deoxyAdenosine-3'-[(2-cyanoethyl)-(N,N-diisopropyl)]-phosphoramidite
|
| Formula: C55H65F3N9O8P |
M.W.: 1068.14 |
F.W.: 427.40 |
Diluent: Anhydrous Acetonitrile |
| Coupling: Amino-Modifier C6 dA reacts in a manner identical to normal phosphoramidites. To prevent side reactions, synthesize using acetyl-protected dC. See Deprotection for futher details. |
| Deprotection: No changes needed from standard method recommended by synthesizer manufacturer. The TFA protecting group is removed during standard ammonium hydroxide deprotection. A minor side reaction during ammonia deprotection can lead to irreversibly capping 2-5% of the amine. This could be significant if multiple additions of the modifier are made. To prevent the reaction, synthesize using acetyl-protected dC and deprotect in 30% ammonia/40% methylamine 1:1 (AMA) at 65°C for 15 minutes. |
| Storage: Refrigerated storage, maximum of 2-8°C, dry |
| Stability in Solution: 2-3 days |
SEQUENCE MODIFIERS
Sequence Modifiers are designed for use in automated synthesis. The carboxy-dT is hydrolyzed during deprotection and can be coupled directly to a molecule containing a primary amino group by a standard peptide coupling or via the intermediate N-hydroxysuccinimide (NHS) ester. Amino-Modifier dA, Amino-Modifier dC, Amino-Modifier dG and both Amino-Modifier dT products can be added in place of a dA, dC, dG and dT residue, respectively, during oligonucleotide synthesis. Corresponding Amino-Modifier supports can replace their respective deoxynucleoside supports. After deprotection, the primary amine on the C6 analogues is separated from the oligonucleotide by a spacer arm with a total of 7 -10 atoms and can be labelled or attached to an enzyme. The C2 analogue is more suitable for the attachment of molecules designed to react with the oligonucleotide.
If you cannot find the answer to your problem then please contact us or telephone +44 (0)1954 210 200